2-oxobutyrate p-toluyl hydrazone di-n-butyltin complex as well as preparation method and application thereof
A technology of di-n-butyltin and di-n-butyltin oxide with toluylhydrazone, which is applied in tin organic compounds, pharmaceutical formulations, organic chemical methods, etc., can solve problems such as undiscovered compounds, and achieve simple preparation methods, The effect of high cancer activity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
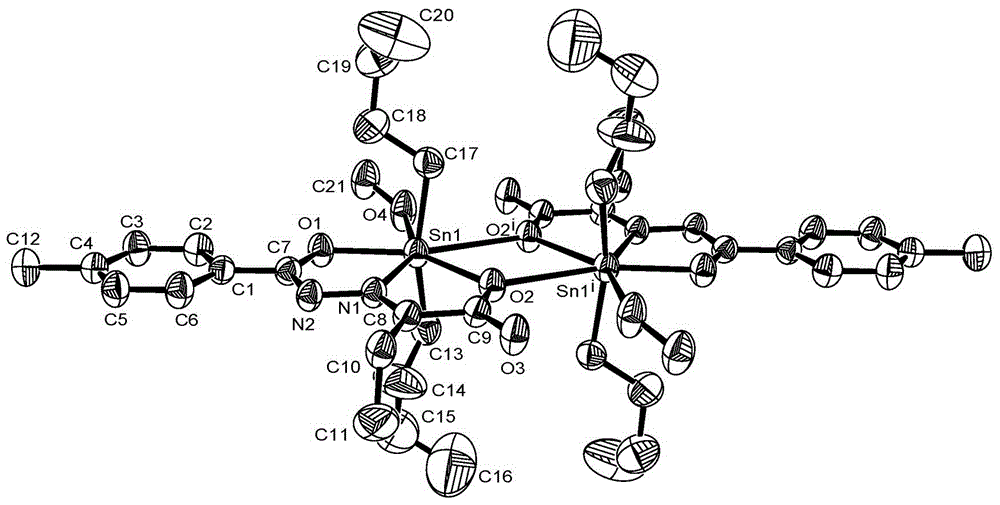
[0042] Preparation of 2-carbonylbutanoic acid p-methylbenzoylhydrazone di-n-butyltin complex:
[0043] Add 0.249g (1.0mmol) di-n-butyltin oxide, 0.150g (1.0mmol) p-toluylhydrazide, 0.112g (1.1mmol) 2-butanuonic acid and 15mL The solvent is anhydrous methanol, and reacted at a temperature of 45~65°C for 8 hours, cooled, filtered, and controlled to volatilize and crystallize the solvent at 20~35°C to obtain a yellow transparent crystal, which is 2-carbonylbutyric acid p-formaldehyde Di-n-butyltin complexes of benzoylhydrazone. Yield: 84.9%. Melting point: 86~88°C (dec).
[0044] Elemental analysis (C 42 h 68 N 4 o 8 sn 2 ): Calculated: C 50.73, H 6.89, N 5.63; Found: C 50.79, H 6.90, N 5.65.
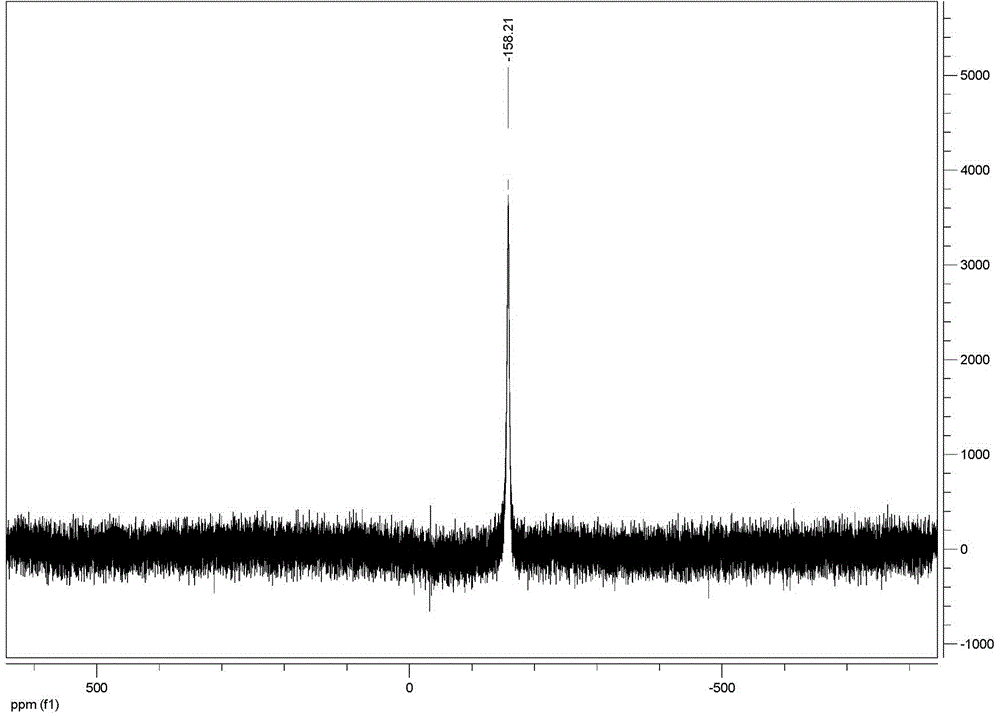
[0045] FT-IR (KBr, ν / cm -1 ): 3527, 2960, 2922, 2858, 1608, 1581, 1487, 1460, 1390, 1334, 1296, 1261, 1193, 1176, 1155, 1058, 837, 862, 707, 684, 626, 8, 603 464, 443.
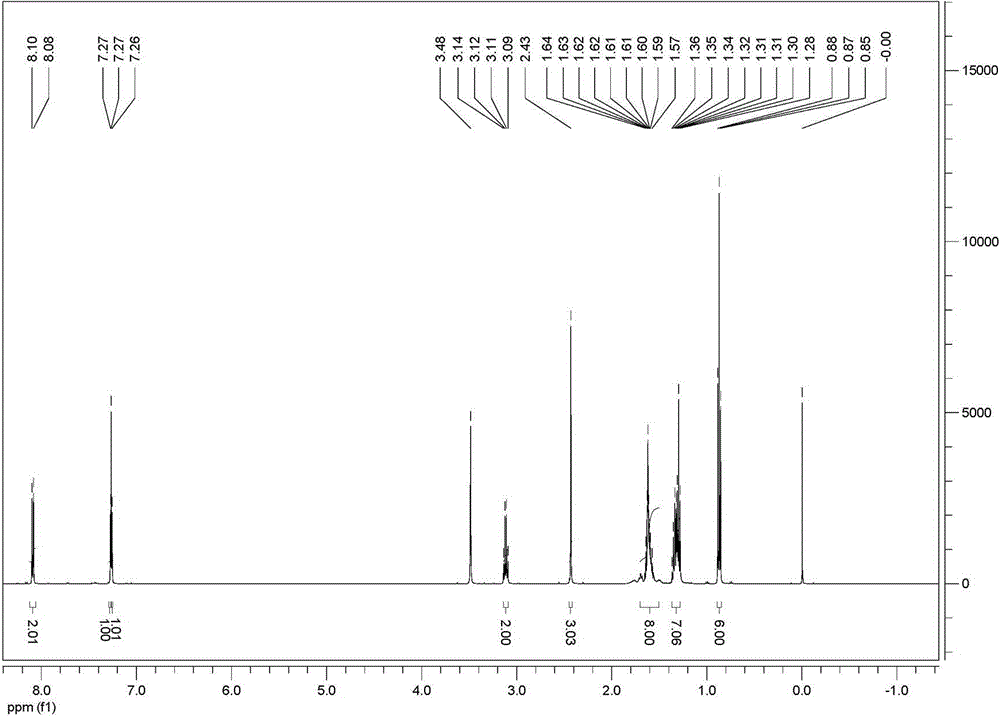
[0046] 1 H NMR (500 MHz, CDCl 3 , δ / ppm): 8.09 (d, J =8.2 Hz, 2H), 7.27 (s, 1H),7.26 (s, 1H), 3.48 (s, 3...
Embodiment 2
[0051] Preparation of 2-carbonylbutanoic acid p-methylbenzoylhydrazone di-n-butyltin complex:
[0052] Add 0.249g (1.0mmol) di-n-butyltin oxide, 0.150g (1.0mmol) p-toluylhydrazide, 0.107g (1.05mmol) 2-butanuonic acid and 35mL The solvent is anhydrous methanol, reacted for 5 hours at a temperature of 45~65°C, cooled, filtered, and controlled solvent volatilization and crystallization at a temperature of 20~35°C to obtain a yellow transparent crystal, which is 2-carbonylbutyric acid p-methyl Di-n-butyltin complexes of benzoylhydrazone. Yield: 85.5%. Melting point: 86~88°C (dec).
[0053] Elemental analysis (C 42 h 68 N 4 o 8 sn 2 ): Calculated: C 50.73, H 6.89, N 5.63; Found: C 50.79, H 6.90, N 5.65.
[0054] FT-IR (KBr, ν / cm -1 ): 3527, 2960, 2922, 2858, 1608, 1581, 1487, 1460, 1390, 1334, 1296, 1261, 1193, 1176, 1155, 1058, 837, 862, 707, 684, 626, 8, 603 464, 443.
[0055] 1 H NMR (500 MHz, CDCl 3 , δ / ppm): 8.09 (d, J =8.2 Hz, 2H), 7.27 (s, 1H),7.26 (s, 1H), 3...
Embodiment 3
[0060] Preparation of 2-carbonylbutanoic acid p-methylbenzoylhydrazone di-n-butyltin complex:
[0061] Add 0.249g (1.0mmol) di-n-butyltin oxide, 0.157g (1.05mmol) p-toluylhydrazide, 0.117g (1.15mmol) 2-butanuonic acid and 25mL The solvent is anhydrous methanol, reacted for 24 hours at a temperature of 45~65°C, cooled, filtered, and controlled solvent volatilization and crystallization at a temperature of 20~35°C to obtain a yellow transparent crystal, which is 2-carbonylbutyric acid p-methyl Di-n-butyltin complexes of benzoylhydrazone. Yield: 84.6%. Melting point: 86~88°C (dec).
[0062] Elemental analysis (C 42 h 68 N 4 o 8 sn 2 ): Calculated: C 50.73, H 6.89, N 5.63; Found: C 50.79, H 6.90, N 5.65.
[0063] FT-IR (KBr, ν / cm -1 ): 3527, 2960, 2922, 2858, 1608, 1581, 1487, 1460, 1390, 1334, 1296, 1261, 1193, 1176, 1155, 1058, 837, 862, 707, 684, 626, 8, 603 464, 443.
[0064] 1H NMR (500 MHz, CDCl 3 , δ / ppm): 8.09 (d, J =8.2 Hz, 2H), 7.27 (s, 1H),7.26 (s, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com