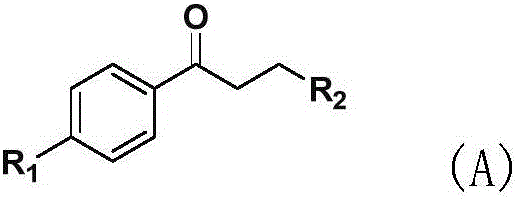
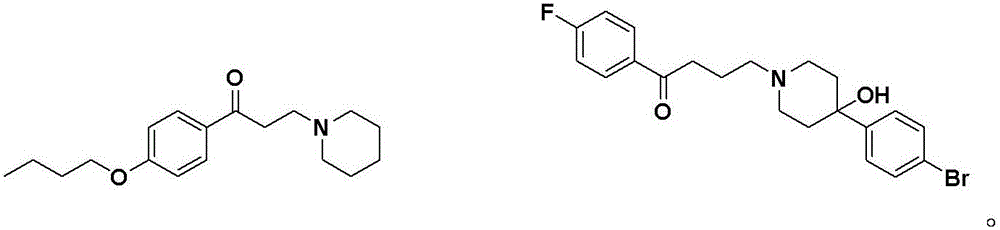
New application of phenyl ketone medicines
A drug and application technology, applied in the field of medicinal chemistry, can solve problems such as unclear role of structural domains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
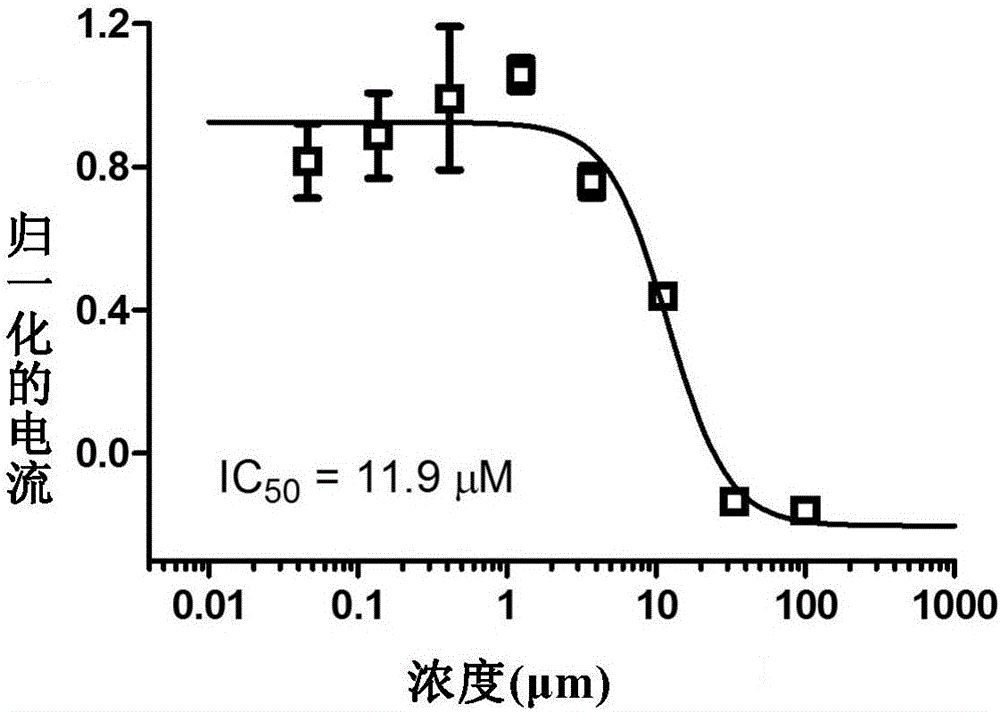
[0098] Using the method described above, compounds 1 and 2 were subjected to IC 50 Inhibitory Activity Test.
[0099] The results are shown in Table 1 below: the compounds 1, 2, 3 and 4 of the present invention are all transient receptor potential channel protein (TRPA1) activity inhibitors, and the compounds 1 and 2 have significant inhibitory effect on the activity of TRPA1.
[0100] Table 1. Compound (1-2) inhibitory activity data to TRPA1 (IC 50 ,μM)
[0101]
Embodiment 2
[0103] pill box
[0104] Prepare following a kind of medicine box, described medicine box comprises:
[0105] (1) a first container, and a first pharmaceutical preparation (tablet) located in said container, which preparation contains the following active ingredients;
[0106]
[0107] (2) a second container, and a first pharmaceutical preparation (such as a tablet) located in said container, which preparation contains the following active ingredients;
[0108]
[0109] and (3) instruction manual.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com