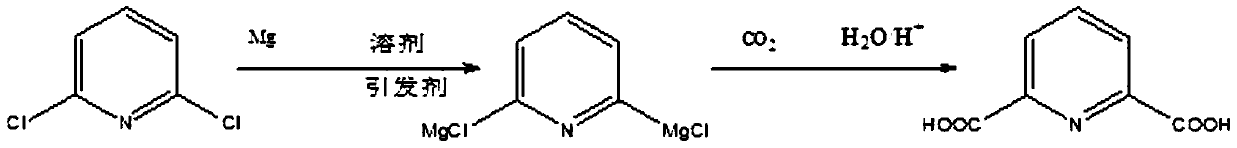
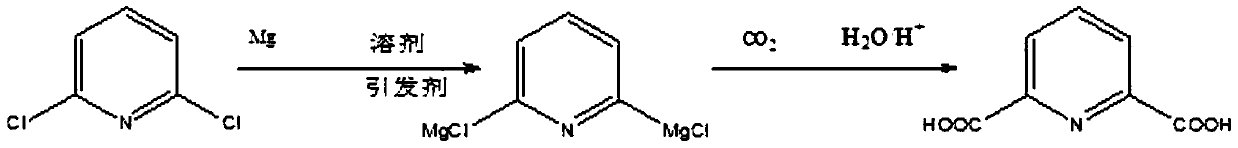
A method for synthesizing 2,6-pyridinedicarboxylic acid
A technology of dipicolinic acid and dichloropyridine, which is applied in the development of pharmaceutical intermediates and the field of pesticides, can solve the problems of high cost and high price, and achieve the effect of low environmental pollution, great cost advantage and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dissolve 148g (1mol) of 2,6-dichloropyridine in 721g (10mol) of tetrahydrofuran solution, add 60g (2.5mol) of magnesium powder, pass through nitrogen to isolate the air, and heat the solution to 55°C while stirring (ie 2, The reaction temperature of 6-dichloropyridine and active metal is 55°C), add 0.55g (0.005mol) of initiator dibromoethane, stir and react for 6 hours (that is, the reaction time of 2,6-dichloropyridine and active metal is 6 hours), the solution after the reaction is transparent; cool down to -15°C, slowly pass in excess dry carbon dioxide gas, judge the reaction according to the flow rate of carbon dioxide, judge the end of the reaction when the amount of carbon dioxide entering is the same as that of the tail gas, and acidify the solution after the end , filtered to remove tetrahydrofuran to obtain 2,6-pyridinedicarboxylic acid with a yield of 94.5% and a purity of 97% after extraction,
Embodiment 2
[0023] Except that before adding the initiator, the solution was heated to 30°C (that is, the reaction temperature between 2,6-dichloropyridine and the active metal was 30°C), the remaining reaction conditions were exactly the same as in Example 1. After the reaction, 2, 6-pyridinedicarboxylic acid, the yield is 92%, and the purity after extraction is 75.2%.
Embodiment 3
[0025] Except that before adding the initiator, the solution was heated to 60°C (that is, the temperature for the interaction between 2,6-dichloropyridine and the active metal was 60°C), the rest of the reaction conditions were exactly the same as in Example 1. After the reaction, 2 , 6-pyridinedicarboxylic acid, the yield was 93.8%, and the purity after extraction was 94.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com