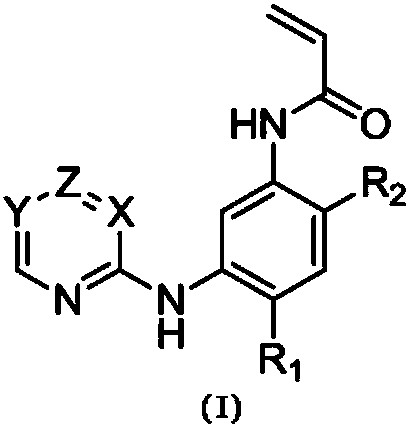
2-Arylaminopyridine, pyrimidine or triazine derivatives and their preparation and use
A pyridine and pyrimidine technology, used in drug combinations, pharmaceutical formulations, active ingredients of heterocyclic compounds, etc., can solve problems such as inability to achieve patient drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] Example 1: N-(5-((4-(benzo[d]isoxazol-3-yl)pyrimidin-2-yl)amino)-4-(methoxy)-2-((2- (Dimethylamino)ethyl)(methyl)amino)phenyl)acrylamide (compound 14a)
[0144]
[0145] Compound 13a (40 mg, 0.14 mmol) was dissolved in dichloromethane (10 mL) and tert-butanol (1 mL), and 1-(3-dimethylaminopropyl)-3-ethylcarbadiene was added under ice-salt bath cooling Imine hydrochloride (EDCI, 35mg, 0.28mmol), triethylamine (19mg, 0.28mmol) and acrylic acid (13mg, 0.28mmol) were added, and the mixture was heated to room temperature for 2h. A saturated potassium carbonate solution was added to the reaction solution, stirred for 10 min, and separated, the organic phase was dried, evaporated to dryness, and purified by preparative chromatography to obtain 8 mg of a light yellow solid. 1 H NMR (400MHz, CDCl 3 ):δ10.23(s,1H),9.50(s,1H),8.69(d,J=5.0Hz,1H),8.57(d,J=8.0Hz,1H),7.68-7.57(m,4H) ,7.38-7.29(m,1H),6.85(s,1H),6.45-6.28(m,2H),5.71(m,1H),3.93(s,3H),2.96-2.88(m,2H),2.75 (s,3H),2....
Embodiment 2
[0155] Example 2: N-(5-((4-(benzo[d]isoxazol-3-yl)pyrimidin-2-yl)amino)-4-methoxy-2-(4-methylpiper (oxin-1-yl)phenyl)acrylamide (compound 14b)
[0156]
[0157] Compound 14b was prepared in the same manner as compound 14a, except that compound 13a was replaced with compound 13b. 1 HNMR (400MHz, CDCl 3 ):δ9.45(s,1H),8.68(d,J=5.0Hz,1H),8.56-8.54(m,2H),7.68-7.58(m,4H),7.39-7.35(m,1H), 6.85(s,1H),6.44-6.23(m,2H),5.78(d,J=9.1Hz,1H),3.93(s,3H),2.99-2.98(m,4H),2.69(m,4H) ,2.45(s,3H); MS(ESI)(m / z):[M+H] + 486.2.
[0158] 1-(4-bromo-5-methoxy-2-nitrophenyl)-N,N-dimethylpiperidin-4-amine (compound 11c)
[0159]
[0160] Compound 11c was prepared in the same manner as compound 11a except that N,N,N'-trimethylethylenediamine was replaced by N,N-dimethylpiperidin-4-amine. 1 H NMR (400MHz, CDCl 3 )δ8.22(s,1H),6.51(s,1H),3.97(s,3H),3.38(d,J=12.4Hz,2H),2.93-2.87(m,2H),2.45-2.31(m ,7H),1.95-1.92(m,2H),1.84-1.74(m,2H); MS(ESI)(m / z):[M+H] + 358.1.
[0161] 4-(Benzo[d]isoxazol-3-y...
Embodiment 3
[0167] Example 3: N-(5-((4-(benzo[d]isoxazol-3-yl)pyrimidin-2-yl)amino)-2-(4-(dimethylamino)piperidine-1 -yl)-4-methoxyphenyl)acrylamide (compound 14c)
[0168]
[0169] Compound 14c was prepared in the same manner as compound 14a, except that compound 13a was replaced by compound 13c. 1 HNMR (400MHz, CDCl 3 ):δ9.39(s,1H),8.65(d,J=5.0Hz,1H),8.52(d,J=7.9Hz,1H),8.41(s,1H),7.75-7.53(m,4H) ,7.36(t,J=7.4Hz,1H),6.76(s,1H),6.37-6.36(m,2H),5.78-5.77(m,1H),3.92(s,3H),3.18-3.15(m ,2H),2.81-2.76(m,3H),2.67(s,6H),2.25-2.22(m,2H),1.98-1.96(m,2H); MS(ESI)(m / z):[M +H] + 514.3.
[0170] (S)-1-(1-(4-bromo-5-methoxy-2-nitrophenyl)pyrrolidin-2-yl)-N,N-dimethylmethylamine (compound 11d)
[0171]
[0172] Except for replacing N,N,N'-trimethylethylenediamine with (S)-N,N-dimethyl-1-(pyrrolidin-2-yl)-methanamine, the same as compound 11a Preparation of compound 11d. 1 H NMR (400MHz, CDCl 3 ):δ8.12(s,1H),6.71(s,1H),3.95(s,3H),3.62-3.55(m,1H),2.72-2.61(m,2H),2.49-2.23(m,8H ),2.09-1.71(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com