Chitosan grafted vanilloyl-based derivative and preparation method and application thereof
A vanillyl derivative and vanillyl technology, applied in the field of chitosan grafted vanillyl derivatives and its preparation, can solve problems affecting sustainable agricultural development, human health, food safety and environmental pollution, and overcome water pollution. Poor solubility, expanding application fields, and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
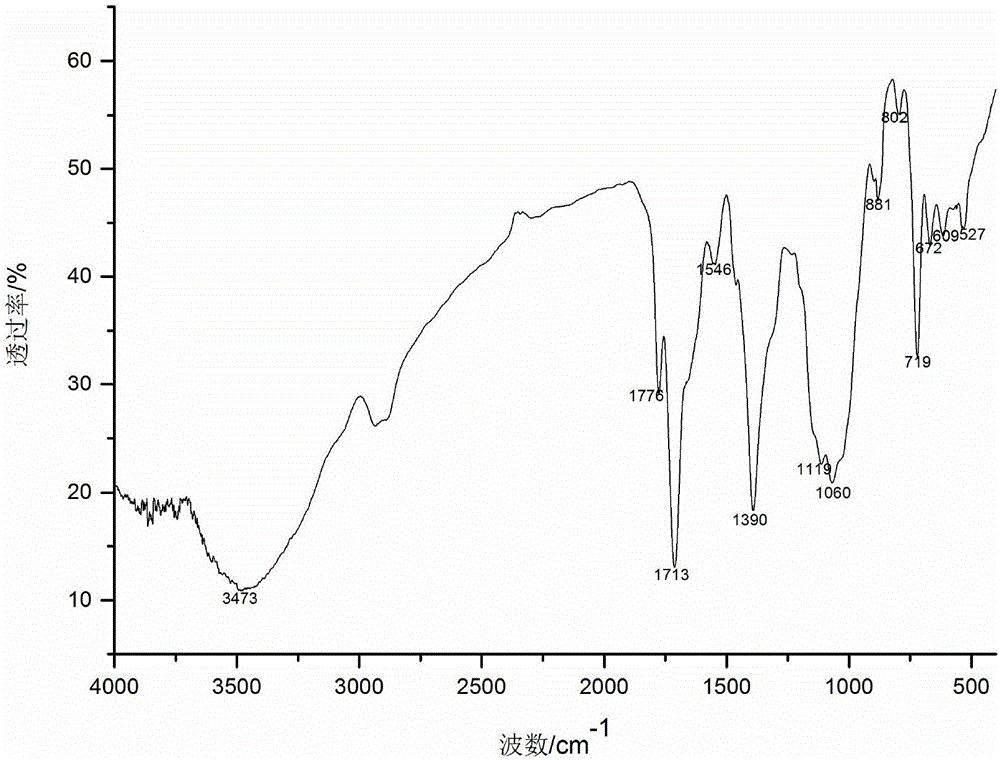
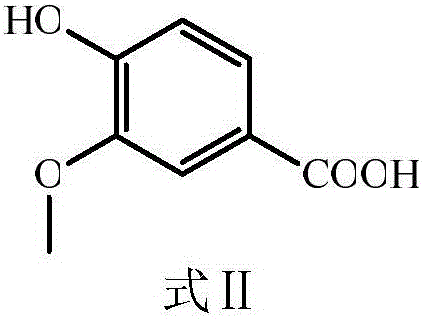
[0024] The preparation of embodiment 1 vanillyl chloride
[0025] Put the obtained carboxylic acid product in a 250mL round-bottomed flask, add 10mL thionyl chloride, drop 3 drops of dimethylformamide (DMF) as a catalyst and solvent, heat and reflux at 40°C for 8h, and then evaporate through rotary evaporation Excessive thionyl chloride, the resulting product is vanillyl chloride.
Embodiment 2
[0026] The preparation of embodiment 2 amino protection 6-O-vanillyl chitosan
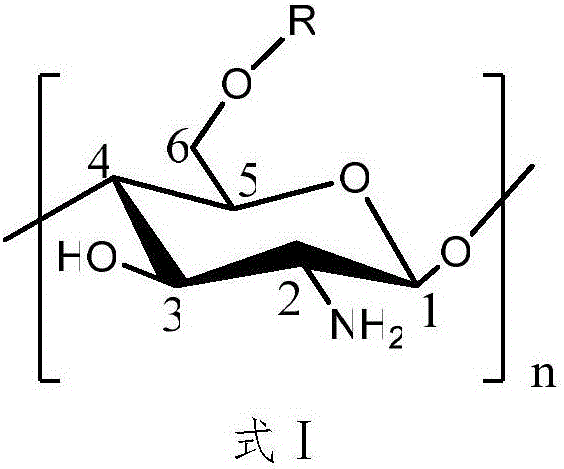
[0027] Disperse 2g of N-phthaloyl chitosan in 40mL of N,N-dimethylformamide, put it in a magnetic heating stirrer, and stir at 60°C for 30min. After the temperature drops to room temperature, dropwise Add 37mL vanillyl chloride, react at 35°C for 10h, pour the reaction solution into 4 times the volume of absolute ethanol, precipitate at 4°C overnight, wash and centrifuge 3 times with absolute ethanol, and freeze-dry the solid to obtain amino-protected 6- O-vanilloyl chitosan.
Embodiment 3
[0028] The preparation of embodiment 3 6-O-vanillyl chitosan
[0029] Put 2g of amino-protected vanillyl chloride chitosan into a 500mL flask, add pyridine and hydrazine hydrate, reflux at 80°C for 6h, pour the reaction solution into 4 times the volume of absolute ethanol, precipitate and centrifuge, and thoroughly wash the solid with absolute ethanol Wash, collect the solid, and finally freeze-dry to obtain 6-O-vanillyl chitosan.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com