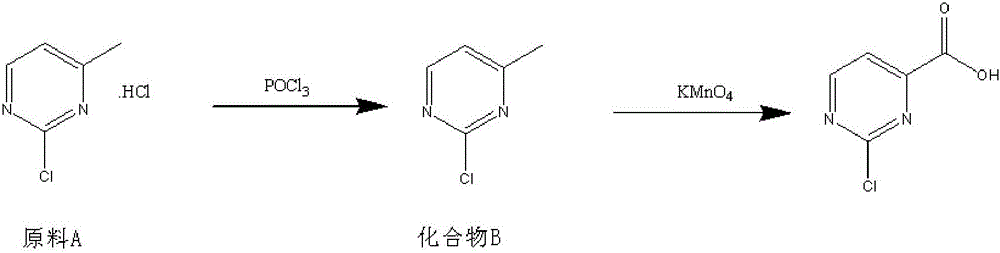
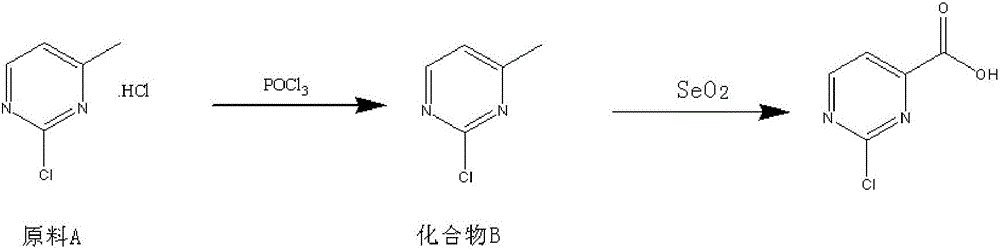
Method for preparing 2-chloropyrimidine-4 formic acid
A technology of chloropyrimidine and methylpyrimidine hydrochloride, applied in the field of compound synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 4.09kg of phosphorus oxychloride and 1.6Kg of raw material A to a 10L reaction bottle, heat to 80 degrees; add 2.22kg of triethylamine dropwise (slow and easy to block), turn off the heating, and maintain the temperature by exothermic reaction ; After dripping, react at 100-105°C for 4h, monitored by TLC. After the reaction, the temperature was lowered to 30° C. for post-treatment.
[0018] Post-treatment: slowly pour the reaction solution into 40L of crushed ice to decompose phosphorus oxychloride; add 2L of dichloroethane to disperse the reaction system to facilitate pouring out the material, and then adjust the pH to 6-7 with 30% sodium hydroxide; Add 4L of ethyl acetate to extract 3 times, combine the extracted and separated organic phases, decolorize with dry activated carbon, filter with suction, and spin dry to obtain 970g of reddish-brown solid product. Test results: melting point around 45 degrees, HPLC: 220nm, 60:40, 97.6% theory: 1403g, yield: 69.1%.
...
Embodiment 2
[0024] Add 4.09kg of phosphorus oxychloride and 1.6Kg of raw material A to a 10L reaction bottle, heat to 80 degrees; add 2.22kg of triethylamine dropwise (slow and easy to block), turn off the heating, and maintain the temperature by exothermic reaction ; After dripping, react at 100-105°C for 4h, monitored by TLC. After the reaction, the temperature was lowered to 30° C. for post-treatment.
[0025] Post-treatment: slowly pour the reaction solution into 40L of crushed ice to decompose phosphorus oxychloride; add 2L of dichloroethane to disperse the reaction system to facilitate pouring out the material, and then adjust the pH to 6-7 with 30% sodium hydroxide; Add 4L of ethyl acetate to extract 3 times, combine the extracted and separated organic phases, decolorize with dry activated carbon, filter with suction, and spin dry to obtain 970g of reddish-brown solid product. Test results: melting point around 45 degrees, HPLC: 220nm, 60:40, 97.6% theory: 1403g, yield: 69.1%.
...
Embodiment 3
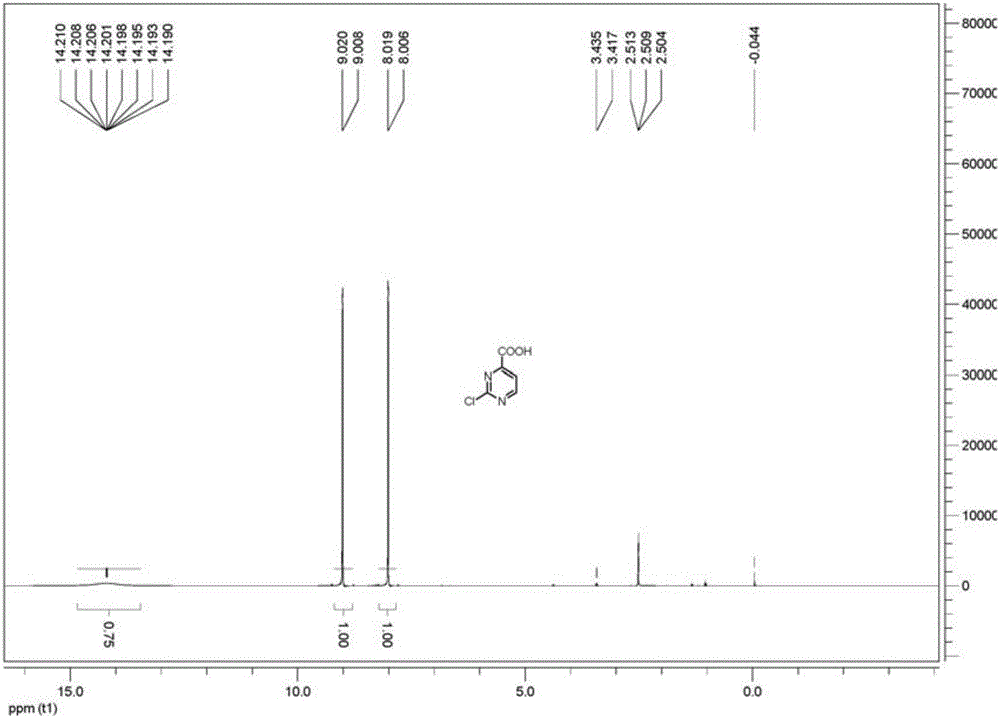
[0031] Combine the concentrated and dried products in Example 1 and Example 2, add 3L*2 times of methanol to reflux to extract the product, concentrate the methanol to semi-dryness, cool and filter to obtain 250g of the product, and concentrate and dry the mother liquor to obtain 60g of the crude product, with a yield of 44%. The spectrum of the measured product is as figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com