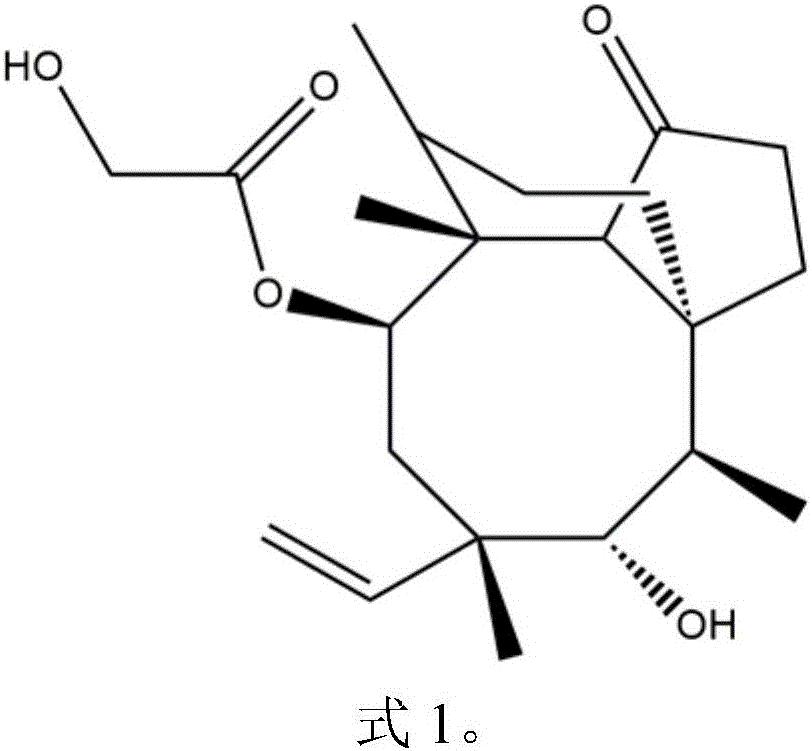
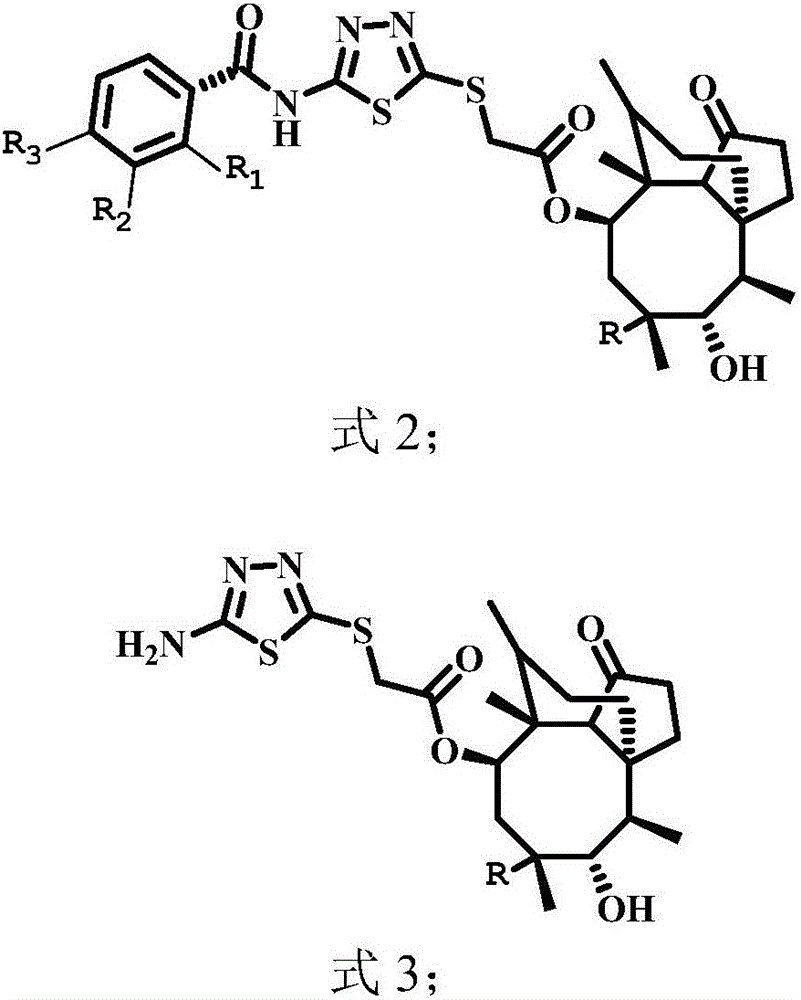
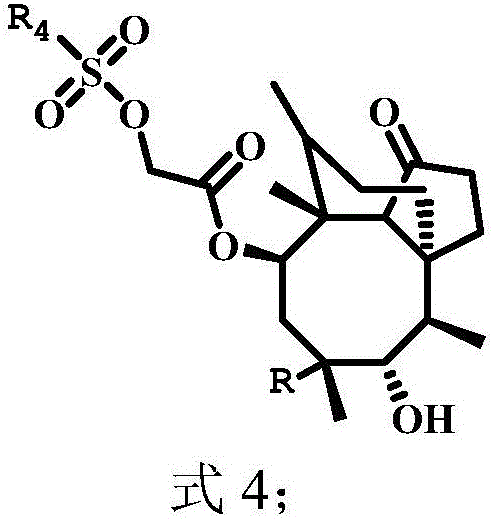
Pleuromutilin compounds with thioether side chains as well as preparation method and application of pleuromutilin compounds
A technique for pleuromutilin and compound, which is applied in the field of pleuromutilin compounds and their preparation, can solve the problem of rare drug-resistant bacteria and the like, and achieve the effect of good in vitro anti-mycoplasma activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Synthesis of 14-O-[2-amino-1,3,4-thiadiazol-5-yl]mercaptoacetyl muulin (compound 18)
[0043] Dissolve 0.905mmol of intermediate I in 10ml of ethyl acetate, add 1.36mmol of sodium iodide and 0.905mmol of potassium carbonate, heat to reflux for 1 hour, add the alkaline solution of 2-amino-5-mercapto-1,3,4-thiadiazole , heated and stirred for 2 hours, poured the reaction solution into a separatory funnel, added chloroform for extraction, took the organic phase and evaporated to dryness, and the obtained crude product was separated and purified by silica gel column chromatography to obtain compound 18: 14-O-[2-amino -1,3,4-Thiadiazol-5-yl]mercaptoacetylmyulin. HR-MS (ESI): Calcd for C24 h 36 N 3 o 4 S 2 (M+H + ):494.2142; Found: 494.2164.
Embodiment 2
[0044] Example 2: Synthesis of 14-O-[2-(2-fluorobenzamido)-1,3,4-thiadiazol-5-yl]mercaptoacetylmyulin (compound 1)
[0045] Dissolve 1 g of compound 18 in 35 ml of ethyl acetate, add 2-fluorobenzoyl chloride equimolar to compound 18, heat and stir at 70°C for 3 h, recrystallize or purify by column chromatography to obtain the target product. The obtained reaction solution was rotary evaporated to dryness, and the mixture was redissolved in dichloromethane. After adding 1 g of 100-200 mesh silica gel for adsorption and drying, 200-300 mesh silica gel was used to pack the column under normal pressure, and the column was separated according to the solvent system determined by TLC. Compound 1 with relatively high purity was obtained with a yield of 85%. HR-MS (ESI): Calcd for C 31 h 39 FN 3 o 5 S 2 (M+H + ): 616.2310; Found: 616.2331.
Embodiment 3
[0046] Example 3: Synthesis of 14-O-[2-(3-fluorobenzamido)-1,3,4-thiadiazol-5-yl]mercaptoacetylmyulin (compound 2)
[0047] Compound 2 was obtained by the same method as described in Example 2 except that 3-fluorobenzoyl chloride was used as the starting material, and the yield was 78%. HR-MS (ESI): Calcd for C 31 h 39 FN 3 o 5 S 2 (M+H+): 616.2310; Found: 616.2330.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com