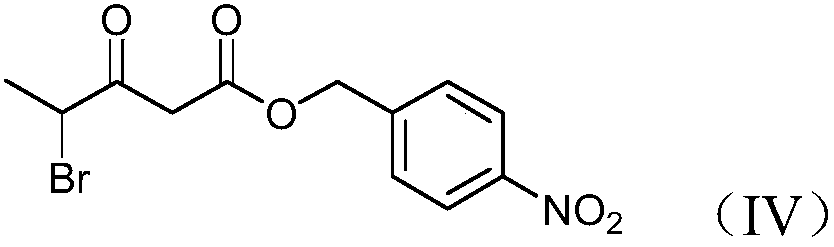
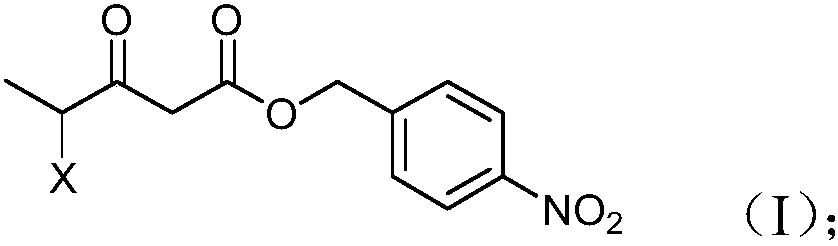
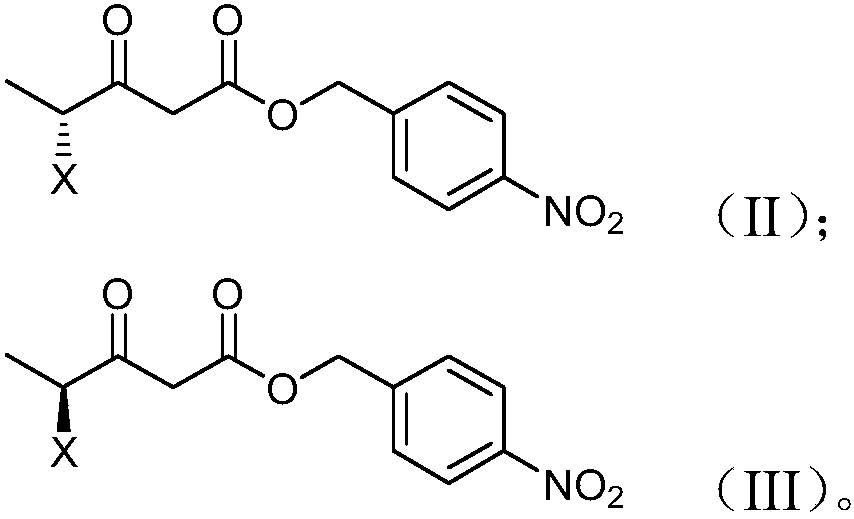
Preparation method and product of 4-halo-3-oxo-pentanoic acid (4-nitrophenyl) methyl ester
A technology of nitrobenzene and oxo, which is applied in the preparation of organic compounds, organic chemical methods, chemical instruments and methods, etc., can solve the problem of high preparation cost of intermediates, and achieve high molecular utilization, simple overall steps, and molecular economy. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Dissolve 108.5kg of 2-chloropropionic acid in 550L of dichloromethane, add 165kg of N,N'-carbonyldiimidazole, and react at a temperature of 18-20°C for 1 hour. After the reaction is complete, cool down to 0°C, add 100kg of magnesium chloride and 239kg of mono-p-nitrobenzyl malonate in sequence, react at 0°C for 15 minutes, then raise the temperature to reflux for 2 hours. After the reaction is over, add hydrochloric acid with a mass fraction of 10%, adjust the pH value to 3-4, let stand and separate layers, add 500L of mass fraction of potassium carbonate aqueous solution to the organic layer to wash, and then use a mass fraction of 10% to the organic layer. sodium chloride aqueous solution to neutrality. After dehydration with anhydrous magnesium sulfate, decolorization with activated carbon, and desolvation under reduced pressure, the feed solution was crystallized at 20-25°C for 1 hour by adding 500L of n-hexane, then centrifuged and dried at 40°C to obtain 250kg of ...
Embodiment 2
[0061] Dissolve 108.5kg of S-2-chloropropionic acid in 500L of chloroform, add 169kg of N,N'-carbonyldiimidazole, and react at a temperature of 0-5°C for 2 hours. After the reaction was complete, 100kg of magnesium fluoride and 240kg of mono-p-nitrobenzyl malonate were added successively, reacted at 0°C for 15min, and then heated to reflux for 5h. After the reaction is finished, add hydrochloric acid with a mass fraction of 5%, adjust the pH value to 3-4, let stand for stratification, add 600L mass fraction of 10% sodium bicarbonate aqueous solution to the organic layer for washing, and then use a mass fraction of 8 to the organic layer. % calcium chloride aqueous solution to neutrality. After the feed liquid is dehydrated with anhydrous sodium sulfate, decolorized with silica gel, and desolvated under normal pressure, add 700L n-heptane to crystallize at 20-25°C for 1 hour, then centrifuge and dry at 35°C to obtain 230kg(s)-4-chloro -3-oxo-valeric acid (4-nitrophenyl) methyl...
Embodiment 3
[0063] Dissolve 110kg of L-2-chloropropionic acid in 500L of dichloromethane, add 170kg of N,N'-carbonyldiimidazole, and react at a temperature of 15°C for 2 hours. After the reaction was complete, 100kg of magnesium fluoride and 240kg of mono-p-nitrobenzyl malonate were added successively, reacted at 20°C for 30min, and then heated to reflux for 3h. After the reaction was finished, add 10% hydrochloric acid by mass fraction, adjust the pH value to 3-4, let stand to separate layers, add 400L mass fraction of 1% sodium hydroxide aqueous solution to the organic layer, and wash the organic layer with a mass fraction of 15% % sodium chloride aqueous solution to neutrality. After dehydration with anhydrous magnesium sulfate, decolorization with silica gel, precipitation at normal pressure, add 550L of petroleum ether to crystallize at 20-25°C for 2 hours, then centrifuge and dry at 30°C to obtain 220kg(L)-4-chloro-3 -Oxo-valeric acid (4-nitrophenyl) methyl ester, the yield is 77%,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com