Quality control method for drug microtox assay system
A quality control method and drug technology, applied in the direction of measuring devices, chemiluminescence/bioluminescence, instruments, etc., can solve the problems of no quality control reference system, low detection accuracy, and difficulty in ensuring accuracy, achieving high sensitivity, High precision and accuracy, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 The establishment of the quality control method of drug microtoxicity test of the present invention
[0030] 1. Establishment method of quality control system
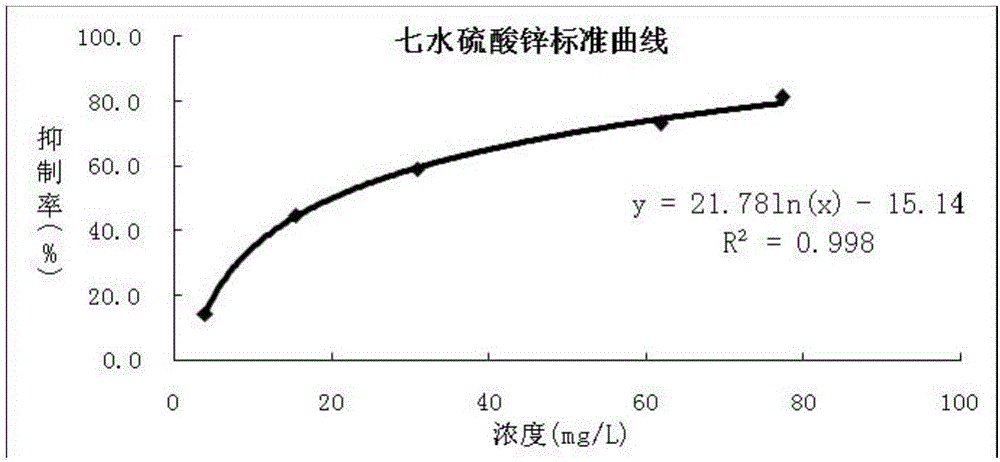
[0031] Precisely weigh ZnSO 4 ·7H 2 O 50.0mg is placed in a 500ml volumetric flask, fully dissolved in ultrapure water, and the volume is constant to obtain 100.0mg·L -1 stock solution. The stock solution was diluted with ultrapure water to obtain a concentration of 5.0 mg·L -1 , 20.0mg·L -1 , 40.0mg·L -1 and 80.0mg·L -1 A series of solutions, and then mix each concentration standard poison with the osmotic pressure adjustment solution at 17:3 as a reference solution for drawing a standard curve;
[0032] In addition, the concentrations of 15.0mg·L can be prepared respectively -1 , 50.0mg·L -1 and 90.0mg·L -1 ZnSO 4 ·7H 2 O solution was mixed with 20% sodium chloride solution at a volume ratio of 17:3 to obtain a series of solutions as quality control sample solutions;
[0033] Vibrio f...
Embodiment 2
[0035] Example 2 Screening of the quality control method of the drug microtoxicity test of the present invention
[0036] 1. Experimental method
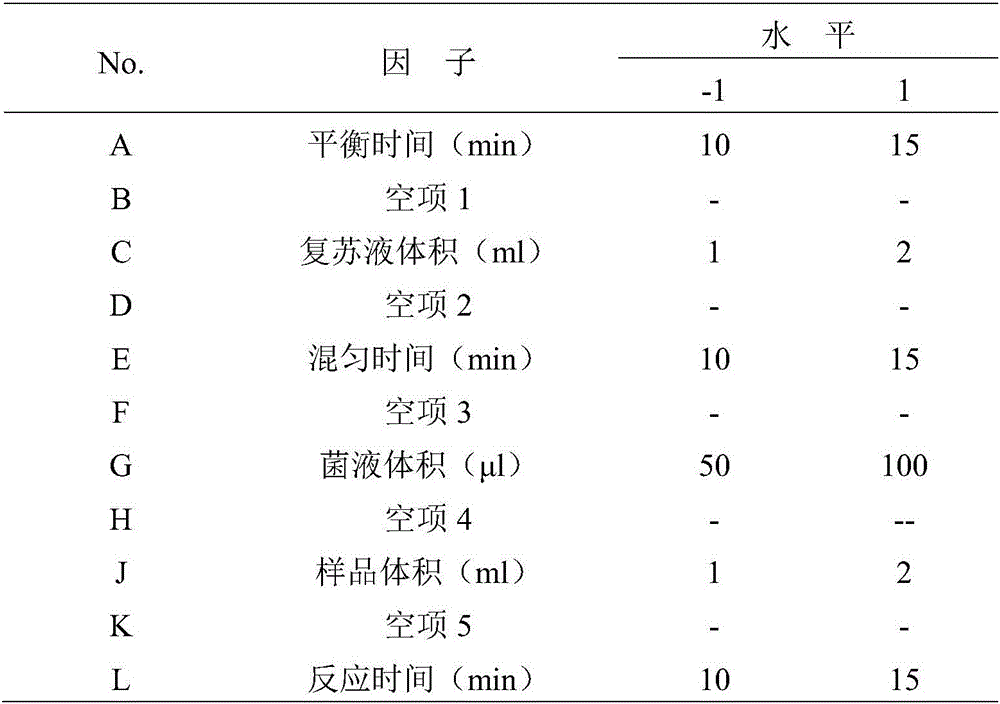
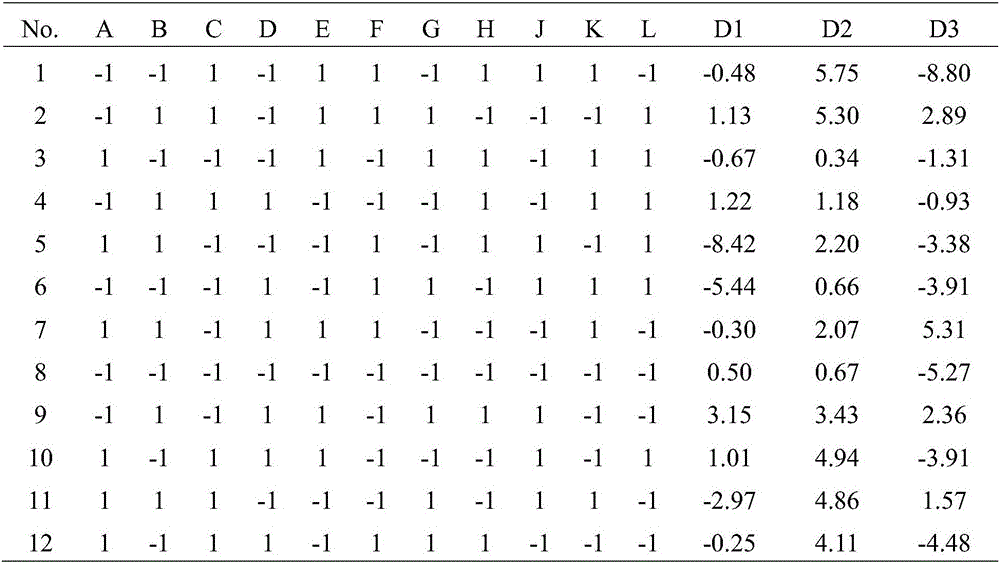
[0037] The six factors in the reaction conditions were investigated comprehensively by Plackett-Burman method. The experimental design with 11 factors and 2 levels was selected, and a total of 12 experiments were carried out, with B, D, F, H, and K as empty items to estimate the experimental error, and A, C, E, G, J, and L respectively representing the equilibrium time, resuscitation fluid For volume, recovery time, bacterial liquid volume, sample volume and reaction time, each factor is taken as a high or low level, and the best combination is selected based on the variance P value of each test group and the mean deviation D value of the luminescence coefficient. All operations using luminescent bacteria were completed at 15°C±1°C.
[0038] Table 1 Plackett-Burman experimental design factors and levels
[0039]
[0040] Accor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com