Refinement method of arotinolol hydrochloride
A technology of alololol hydrochloride and refining method, which is applied in the field of chemical medicine, can solve the problems of lack of solutions, no cost advantage, cumbersome operation, etc., and achieve the effect of simple operation, control of solvent residue, and mild refining process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
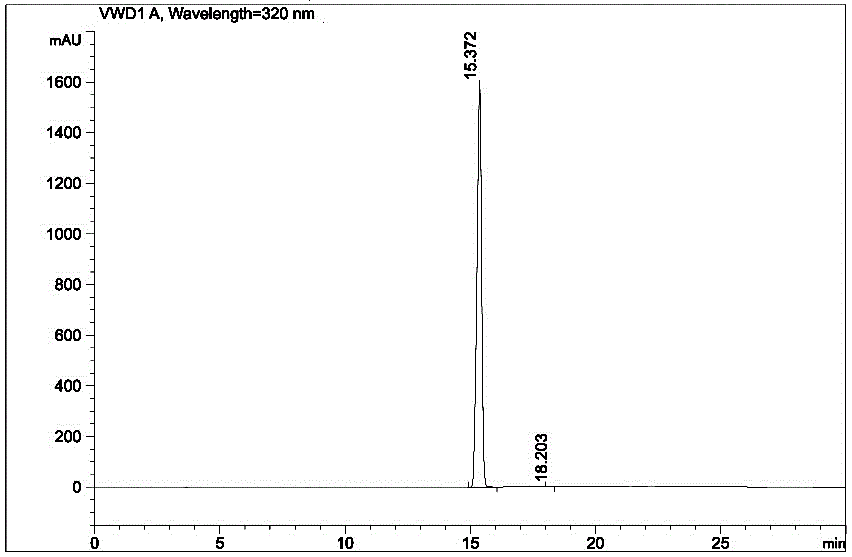
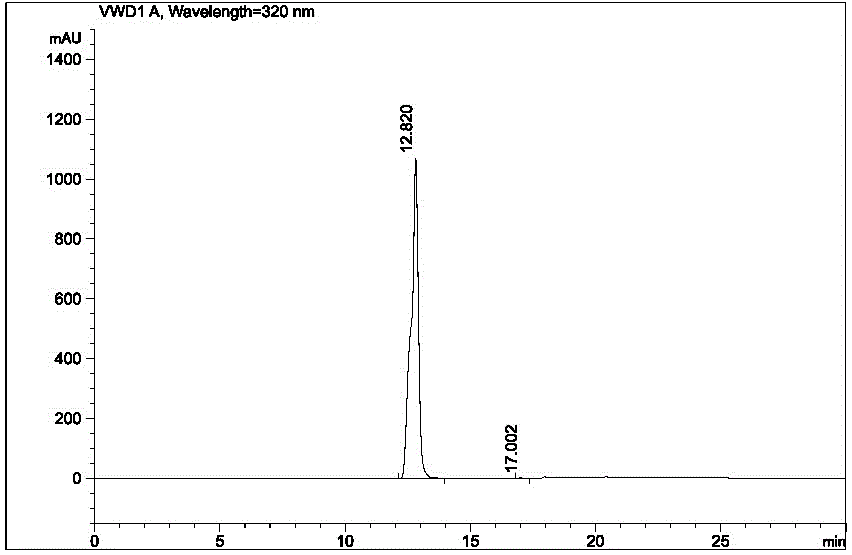
Image
Examples
Embodiment 1
[0030] In a 3 L reaction flask, add 1,100 ml of ethyl acetate, 220 ml of methanol, and 550 ml of purified water, add 55.0 g of arololol hydrochloride crude product under stirring, add weak base sodium carbonate powder to adjust the pH value to 10, and heat up to At about 32°C, stir until dissolved. Let stand to separate the liquids, and extract the aqueous phase with 275 ml of ethyl acetate once more. The organic phases were combined and washed with 500 ml of saturated sodium chloride solution. Add 20.0 g of sodium sulfate and dry for 0.5 h, filter, and concentrate to obtain an oil. Acetonitrile was added thereto, beating for 0.5 h, suction filtered, and dried to obtain 45.7 g of off-white solid, which was Arololol, with a yield of 91%.
[0031] Add 240 ml of methanol and 40.0 g of arololol to a 500 ml reaction flask, heat up and dissolve, add 0.8 g of activated carbon, continue to stir for 0.5 h, and filter with suction. Under stirring, add 20ml of 6 M hydrochloric acid so...
Embodiment 2
[0033] In a 3 L reaction flask, add 1,500 ml of ethyl acetate, 300 ml of methanol, and 750 ml of purified water, add 50.0 g of arololol hydrochloride crude product under stirring, add weak base sodium carbonate powder to adjust the pH value to 11, and heat up to At about 28°C, stir until dissolved. Let stand to separate the liquids, and extract the aqueous phase with 250 ml of ethyl acetate once more. The organic phases were combined and washed with 500 ml of saturated sodium chloride solution. Add 20.0 g of sodium sulfate and dry for 0.5 h, filter, and concentrate to obtain an oil. Methanol was added therein, beating for 0.5 h, suction filtered, and dried to obtain 40.1 g of off-white solid, namely Arololol, with a yield of 88%.
[0034] Add 320 ml of ethanol and 40.0 g of arololol to a 500 ml reaction flask. After heating up and dissolving, add 0.8 g of activated carbon, continue to stir for 0.5 h, and filter with suction. Under stirring, add 20 ml of 6 M hydrochloric aci...
Embodiment 3
[0036] In a 3 L reaction flask, add 1,350 ml of ethyl acetate, 210 ml of methanol, and 780 ml of purified water, add 52.0 g of arololol hydrochloride crude product under stirring, add weak base potassium carbonate powder to adjust the pH value to 11, and heat up to At about 30°C, stir until dissolved. Let stand to separate the liquids, and extract the aqueous phase with 260 ml of ethyl acetate once more. The organic phases were combined and washed with 500 ml of saturated sodium chloride solution. Add 20.0 g of sodium sulfate and dry for 0.5 h, filter, and concentrate to obtain an oil. Add ethanol to it, beat for 0.5 h, filter with suction, and dry to obtain 42.5 g of off-white solid, which is Arololol, with a yield of 90%.
[0037] Add 304 ml of methanol and 38.0 g of alololol to a 500 ml reaction flask, heat up and dissolve, add 1.0 g of activated carbon, continue to stir for 0.5 h, and filter with suction. Under stirring, 19 ml of 6 M hydrochloric acid solution was added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com