4-amino-6-(halo-substituted-alkyl)-picolinates and their use as herbicides
A technology of alkyl and halogen atoms, applied in 4-amino-6-(halogen-substituted alkyl)-picolinate and their use as herbicides, can solve problems such as affecting crop yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
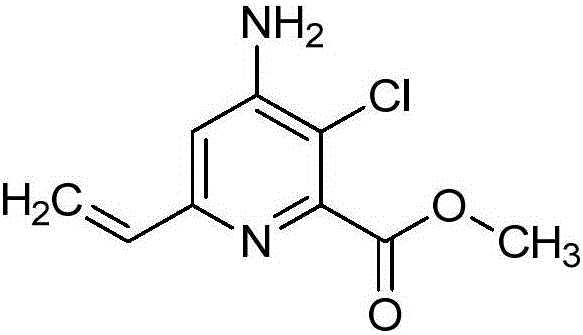
[0095] Embodiment 1: Preparation of methyl 4-amino-3-chloro-6-vinylpicolinate (C1)
[0096]
[0097] Methyl 4-amino-3,6-dichloropicolinate (2.50 g, 11.3 mmol), vinyltributyltin (7.17 g, 22.6 mmol), and bis(triphenylphosphine)palladium(II) dichloride (1.19g, 1.70mmol) in 1,2-dichloroethane (12mL) into a 25-mL vial and seal the cap, which was placed in the Biotage Heat at 120° C. for 30 minutes in a microwave reactor with an external IR-sensor temperature monitored from the side of the vessel. The mixture was loaded directly onto a silica gel column and purified by flash column chromatography (0-40% ethyl acetate / hexanes) to give the title compound (2.03 g, 84%) as a yellow solid: mp 74.5 - 76.5 °C; 1 H NMR (400MHz, CDCl 3 ( s, 2H), 3.98(s, 3H); 13 C NMR (400MHz, CDCl 3 )δ165.8, 154.0, 150.3, 148.0, 135.8, 119.3, 113.5, 107.7, 52.9; ESIMS m / z 213 ([M+H] + ).
[0098] The following compounds were prepared according to the procedure in Example 1:
[0099] Methyl 4-amin...
Embodiment 2
[0111] Example 2: Preparation of methyl 4-amino-3-chloro-6-(1-fluorovinyl)picolinate (F6)
[0112]
[0113] Methyl 4-acetylamino-3,6-dichloropicolinate (0.528g, 2.01mmol), tributyl(1-fluorovinyl)stannane (1.00g, 3.00mmol), and bis(triphenyl A mixture of phosphine)palladium(II) dichloride (0.225g, 0.320mmol) in 1,2-dichloroethane (4mL) was heated at 80°C for 8 hours. The reaction mixture was directly purified by flash column chromatography (0-50% ethyl acetate / hexanes) to give methyl 4-acetamido-3-chloro-6-(1-fluorovinyl)picolinate as an impure mixture (0.512g). To the crude intermediate at 0 °C in methanol (19 mL) was added acetyl chloride (2.90 mL, 37.6 mmol) dropwise. After 30 minutes the ice bath was removed and the reaction mixture was allowed to warm to room temperature and stirred overnight. Methanol was removed in vacuo, and the reaction mixture was neutralized with saturated aqueous sodium bicarbonate. The aqueous solution was extracted three times with ethyl ac...
Embodiment 3
[0114] Example 3: Preparation of methyl 4-amino-3-chloro-5-fluoro-6-(1-fluorovinyl)picolinate (F22)
[0115]
[0116] Methyl 4-amino-3,6-dichloro-5-fluoropicolinate (0.374g, 1.57mmol), tributyl(1-fluorovinyl)stannane (0.590g, 1.76mmol), and bis( A mixture of triphenylphosphine)palladium(II) dichloride (0.175 g, 0.250 mmol) in 1,2-dichloroethane (7 mL) was heated at 70° C. for 3 hours. The reaction mixture was directly purified by flash column chromatography (0-60% ethyl acetate / hexanes). A second purification by reverse phase flash column chromatography (0-40%, 40-40%, 40-100% acetonitrile / water) afforded the title compound (0.0810 g, 21%) as a white powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com