Preparation method of linagliptin
A methanol aqueous solution, tert-butoxycarbonyl technology, applied in organic chemistry and other directions, can solve problems such as amide bond cleavage, and achieve the effect of slow filtration rate and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The method for preparing formula g compound linagliptin, it may further comprise the steps:
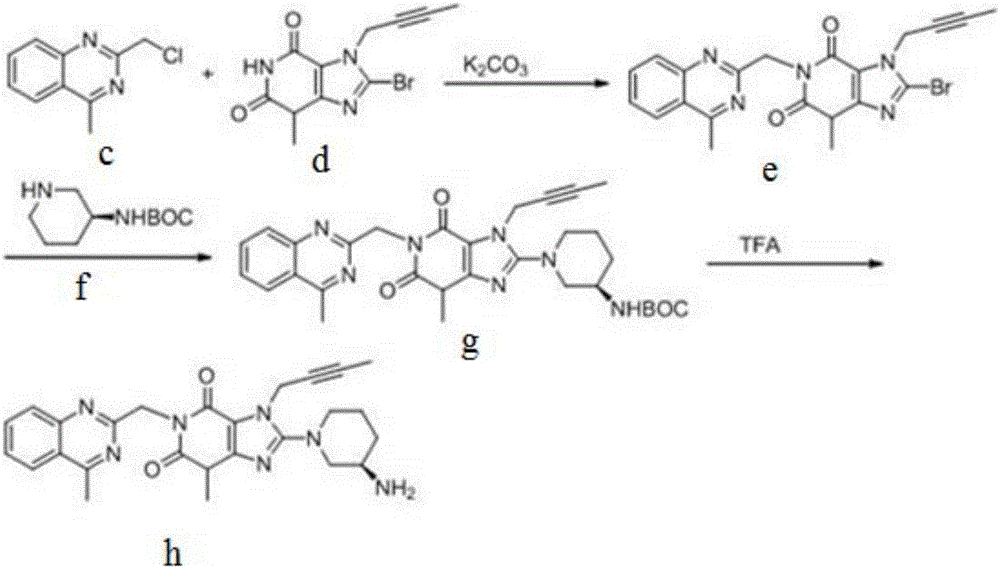
[0041] (1) Synthesis of intermediate (c)
[0042] Reaction of 8-bromo-3-methylxanthine (a) with 1-bromo-2-butyne (b) affords intermediate (c).
[0043]
[0044] Operation steps: Add 908g (3.7mol) 8-bromo-3-methylxanthine (a), 574.1g (4.442mol) N,N-diisopropylethylamine (DIEA) into a 2L three-necked flask, 591.1 g (4.445 mol) of 1-bromo-2-butyne (b), 12 L of acetone. Start stirring, heat to reflux reaction, and the reaction ends after 4-6 hours. The reaction solution was cooled to room temperature, filtered with suction, and the filter cake was washed with 4 L of methanol to obtain a pale yellow solid, which was dried to obtain 1013.7 g of the product with a purity of 95.7% and a yield of 105.9%.
[0045] (2) Synthesis of intermediate (e)
[0046] Intermediate (c) is reacted with 2-chloromethyl-4-methylquinazoline (d) to give intermediate (e).
[0047]
[0048]Operati...
Embodiment 2
[0061] The method for preparing formula g compound linagliptin, it may further comprise the steps:
[0062] (1) Synthesis of intermediate (c)
[0063] Reaction of 8-bromo-3-methylxanthine (a) with 1-bromo-2-butyne (b) affords intermediate (c).
[0064]
[0065] Operation steps: Add 36.75g (0.15mol) 8-bromo-3-methylxanthine (a), 23.26g (0.18mol) N,N-diisopropylethylamine (DIEA) into a 500mL three-necked flask , 21.94g (0.165mol) 1-bromo-2-butyne (b), acetone 350mL. Start stirring, heat to reflux reaction, and the reaction ends after 7h. The reaction solution was cooled to room temperature, filtered with suction, and the filter cake was washed with 100 mL of methanol to obtain a pale yellow solid, which was dried to obtain 52.96 g of the product with a purity of 92.0% and a yield of 118.8%.
[0066] (2) Synthesis of intermediate (e)
[0067] Intermediate (c) is reacted with 2-chloromethyl-4-methylquinazoline (d) to give intermediate (e).
[0068]
[0069] Operation st...
Embodiment 3
[0079] The method for preparing formula g compound linagliptin, it may further comprise the steps:
[0080] (1) Synthesis of intermediate (c)
[0081] Reaction of 8-bromo-3-methylxanthine (a) with 1-bromo-2-butyne (b) affords intermediate (c).
[0082]
[0083] Operation steps: In a 500mL three-necked flask, add 10g (0.04mol) 8-bromo-3-methylxanthine (a), 6.2g (0.048mol) N,N-diisopropylethylamine (DIEA), 6.9g (0.052mol) of 1-bromo-2-butyne (b), 120mL of acetone. Start stirring, heat to reflux reaction, and the reaction ends after 5.5h. The reaction solution was cooled to room temperature, filtered with suction, and the filter cake was washed with 50 mL of methanol to obtain a pale yellow solid, which was dried to obtain 12.9 g of the product with a purity of 93.6 and a yield of 106.3%.
[0084] (2) Synthesis of intermediate (e)
[0085] Intermediate (c) is reacted with 2-chloromethyl-4-methylquinazoline (d) to give intermediate (e).
[0086]
[0087] Operation steps...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com