A method and device for detecting immunogen
A detection method and immunogen technology, applied in the field of biomedicine, can solve problems such as severe cross-reactivity, poor specificity, and inability to distinguish HPV types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] Preparation of monoclonal antibodies
[0088] Antibodies suitable for use in the present invention can be prepared by various techniques known to those skilled in the art. For example, an antigen of the invention may be administered to an animal to induce the production of monoclonal antibodies. For monoclonal antibodies, hybridoma technology can be used to prepare (see Kohler et al., Nature 256; 495, 1975; Kohler et al., Eur.J.Immunol.6:511, 1976; Kohler et al., Eur.J.Immunol. 6:292,1976; Hammerling et al., In Monoclonal Antibodies and T Cell Hybridomas, Elsevier, N.Y., 1981) or can be prepared by recombinant DNA methods (US Patent No. 4,816,567).
[0089] Representative myeloma cells are those that fuse efficiently, support stable high-level production of antibody by selected antibody-producing cells, and are sensitive to culture medium (HAT medium matrix), including myeloma cell lines, such as murine Myeloma cell lines, including those derived from MOPC-21 and MPC-...
Embodiment 1
[0143] Example 1 Specific Identification of HPV18 E7 Protein
[0144] In this example, monoclonal antibodies H11 and F1 are taken as examples to illustrate the specific experimental methods in this example.
[0145]Detect the reaction of monoclonal antibodies H11 and F1 to His-HPV18 E7 and His-HPV16 E7 proteins, and use the multi-antibody coating sandwich ELISA method for identification:
[0146] (1) The purified F1 and H11 antibodies were coated with pH9.6 carbonate buffer (sodium carbonate (Na 2 CO 3 ) 1.59g, sodium bicarbonate (NaHCO 3 ) 2.93g sodium azide (NaN 3 ) 0.2g, adjust the pH value to 9.6 with 10M NaOH, and add distilled water to 1000ml. ) were diluted to respective concentrations of 2 μg / ml and then mixed for coating, 100 μl / well, overnight at 4°C.
[0147] (2) 1x PBST (1xPBS formula is: sodium chloride (NaCl) 5g potassium chloride (KCl) 0.125g potassium dihydrogen phosphate (KH 2 PO 4 )0.125g disodium hydrogen phosphate (Na 2 HPO 4 .12H 2 O) 1.81g of di...
Embodiment 2
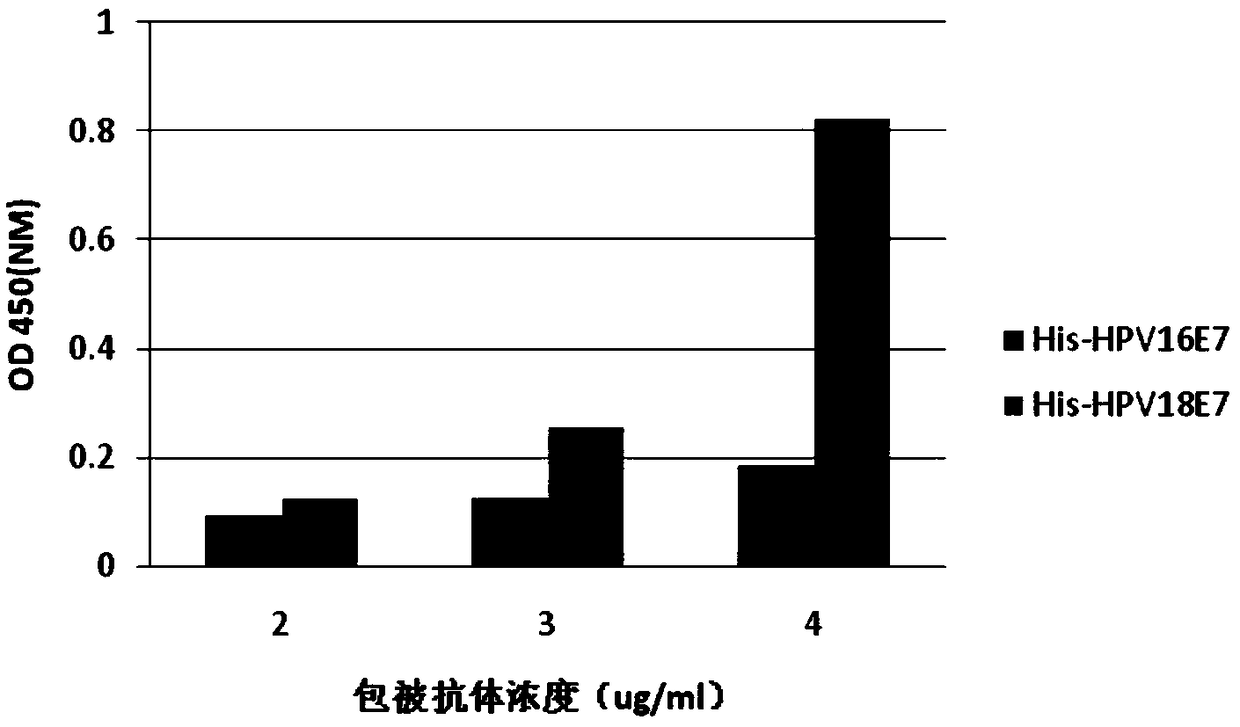
[0163] Example 2 Selection of the Concentration of Coated Antibody in Multi-antibody Coated Sandwich ELISA Detection
[0164] The mixed H11 and F1 antibodies were diluted to 2.0 μg / ml (1.0 μg / ml for H11 and F1), 3.0 μg / ml (1.5 μg / ml for H11 and F1) and 4.0 μg / ml (1.5 μg / ml for H11 and F1, respectively). F1 concentrations were 2.0 μg / ml) for coating, recombinant His-HPV16E7, His-HPV18E7 protein concentrations were 2 μg / ml as antigens, the concentrations of detection antibodies H11-Bio and F1-Bio were 1 μg / ml, each sample Set up two duplicate wells, 100 μl / well, and detect according to the steps of Example 1, such as image 3 As shown, when the total coating concentration of H11 and F1 is 4 μg / ml, that is, when the respective concentrations of the two are 2 μg / ml, negative and positive proteins can be clearly distinguished. Therefore, the total concentration of H11 and F1 antibody coating was selected to be 4 μg / ml for subsequent experiments.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com