A kind of synthetic method of amino acid derivative substituted with β-silicon group
A synthesis method and amino acid technology, which are applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems such as the limited scope of application of substrates, and achieve mild reaction conditions and stereoselectivity. The effect of high sex and strong reaction versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
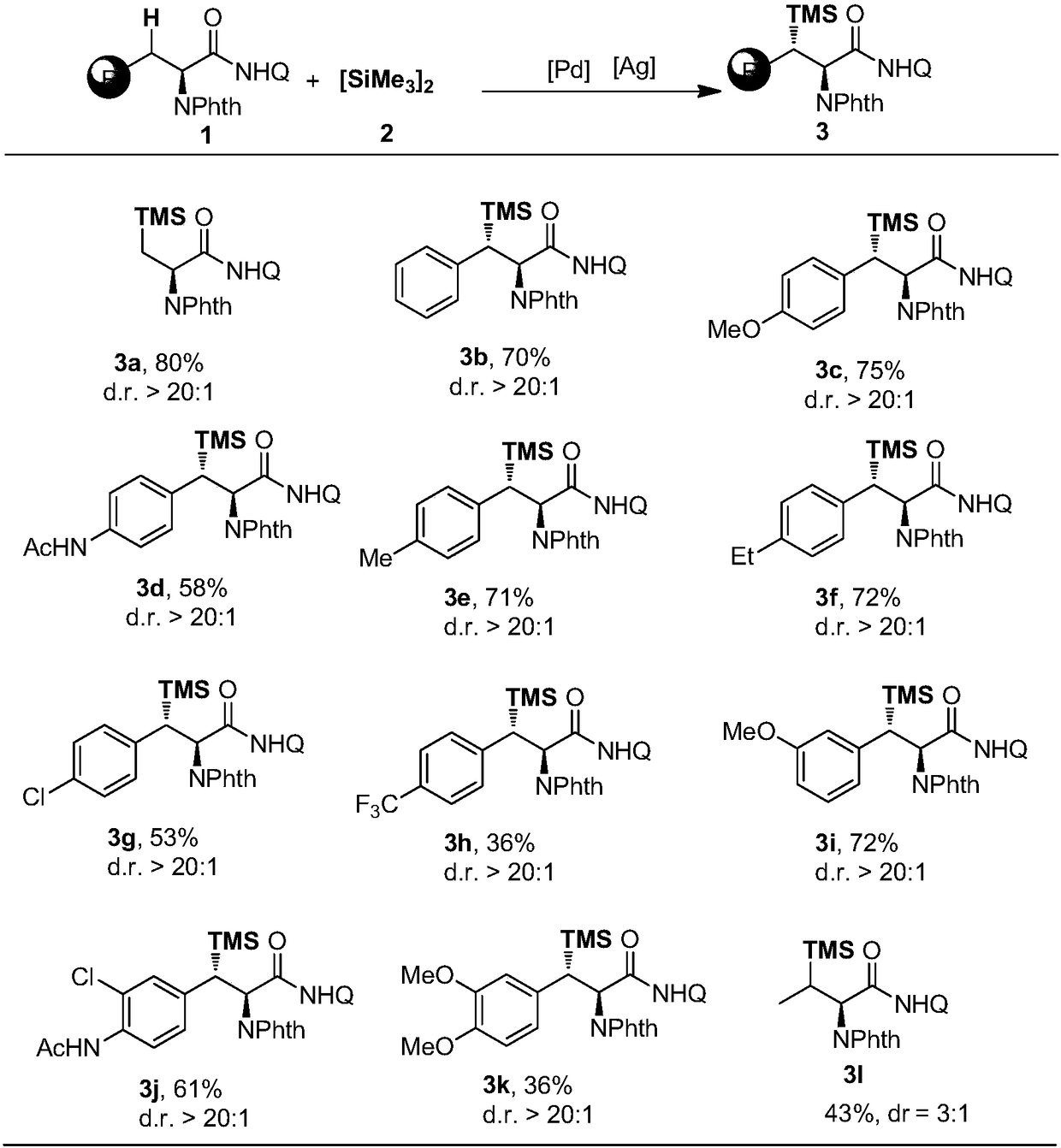
[0035] In the reactor, add 0.1 mmol of alanine whose nitrogen end is protected with phthaloyl group and whose carboxyl end is introduced into 8-aminoquinoline, 0.5 mmol of hexamethyldisilane 2, 0.01 mmol of palladium acetate catalyst, 0.02 Millimoles of L-N-Boc-Val-OH, 2.0 millimoles of silver carbonate and 1 milliliter of tert-butanol were reacted at 140 ° C for 12 hours and then the reaction was completed for post-treatment, and the β-silylation as in structural formula 1 was obtained by silica gel column chromatography Product 3a in 84% yield. 1 H NMR (400MHz, CDCl 3 )δ10.47(br,1H),8.74-8.70(m,2H),8.14(dd,J=8.4,1.2Hz,1H),7.90(dd,J=5.2,3.2Hz,2H),7.75(dd ,J=5.2,3.2Hz,2H),7.50(d,J=4.8Hz,2H),7.42(dd,J=8.0,4.0Hz,1H),5.23(dd,J=12.0,4.8Hz,1H) ,2.08(dd,J=14.4,12.0Hz,1H),1.66(dd,J=14.4,4.8Hz,1H),0.05(s,9H); 13 C NMR (101MHz, CDCl 3 )δ168.41, 168.19, 148.50, 138.77, 136.40, 134.34, 134.21, 132.11, 128.03, 127.45, 123.73, 121.98, 121.73, 116.77, 52.41, 17.31, -1.31.
[0036] The ...
Embodiment 2
[0039] In the reactor, add 0.1 mmol of L-phenylalanine whose nitrogen end is protected with phthaloyl group and carboxyl end with 8-aminoquinoline, 0.5 mmol of hexamethyldisilane 2, 0.015 mmol of palladium acetate Catalyst, 1.5 mmol 2,6-dimethoxy-1,4-benzoquinone, 0.5 mmol silver carbonate and 0.5 mmol lithium acetate and 1 ml 1,4-dioxane, react at 125°C for 12 hours After finishing the reaction, the post-treatment was carried out, and the β-silylation product 3b according to the structural formula 2 was obtained by silica gel column chromatography, and the yield was 71%. 1 H NMR (400MHz, CDCl 3 )δ10.93(br,1H),8.93(dd,J=4.0,1.6Hz,1H),8.82-8.77(m,1H),8.14(dd,J=8.4,1.6Hz,1H),7.69(m ,2H),7.57(td,J=5.2,2.0Hz,2H),7.53(d,J=1.6Hz,1H),7.52(s,1H),7.46(dd,J=8.4,4.0Hz,1H) ,7.25-7.17(m,4H),6.95(ddd,J=8.8,5.2,3.6Hz,1H),5.60(d,J=13.6Hz,1H),3.89(d,J=13.6Hz,1H), 0.02(s,9H); 13 C NMR (100MHz, CDCl 3 )δ168.26, 167.32, 148.81, 139.66, 138.98, 136.27, 134.61, 134.04, 131.37, 128.37, 128.07, ...
Embodiment 3~12
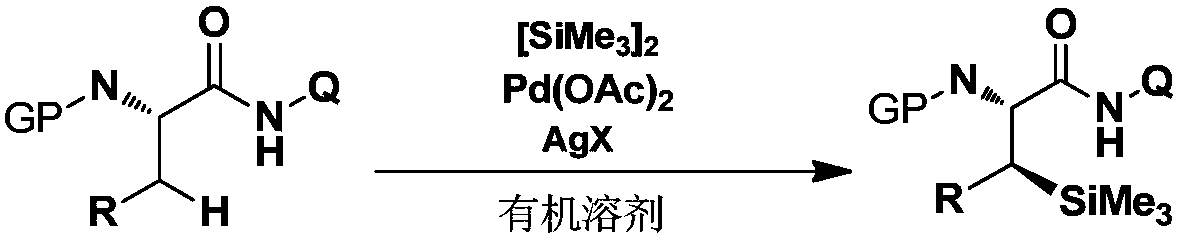
[0043] The operation steps are the same as in Example 2, the difference is that: according to Table 1, the substituent R of the amino acid substrate is changed to obtain different β-silylated phenylalanine derivatives, and the reaction formula is as follows:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com