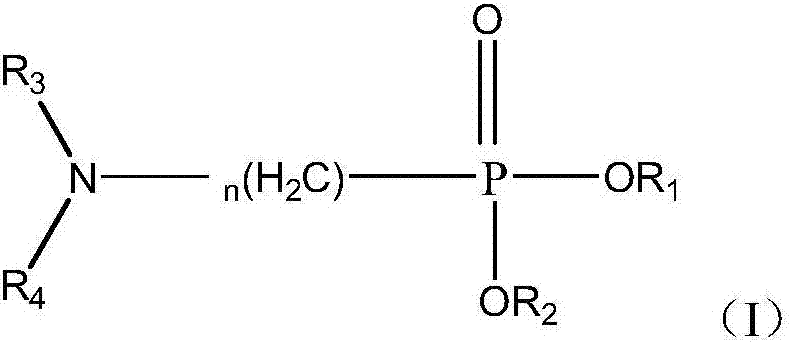
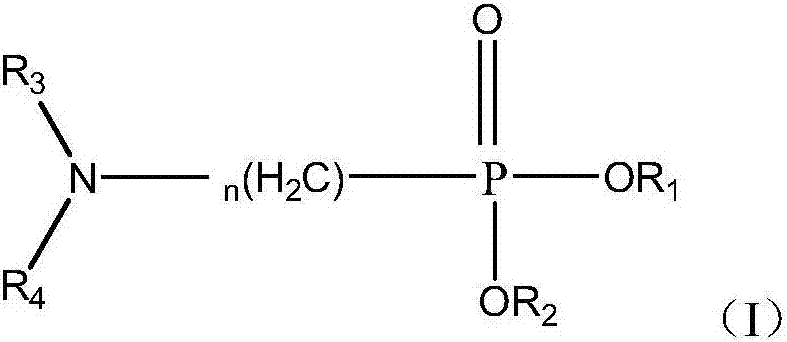
Method for separating cerium-fluorine and thorium
A solid-state separation and extraction technology, which is applied in the direction of improving process efficiency, can solve the problems of harming people's health and polluting the environment, radioactive thorium has not been separated and recovered, and the process is long, so as to achieve stable recycling properties and good stripping phenomenon , easy to synthesize effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0125] In order to further illustrate the solutions of the present invention, specific examples of the present invention are provided to help those skilled in the art understand and implement the present invention, but the present invention is not limited to these examples.
[0126] Reagents and sources
[0127] Diethylhexyl phosphite, di-n-octyl phosphite, di-n-heptyl phosphite, di-isooctyl phosphite, aviation kerosene and TBP were purchased from Shanghai Laish Chemical Co., Ltd.
[0128] Paraformaldehyde, di-n-hexylamine, diisobutylamine, n-butylamine, diisooctylamine, dodecylamine, isooctylamine, dimethylamine, toluene, xylene, p-toluenesulfonic acid, and heptane purchased from Aladdin Reagent Co., Ltd.
[0129] Cyanex 923 and 2-methylheptanol were purchased from Shanghai Cyanex Chemical Co., Ltd.
[0130] Feed liquid, washing liquid and stripping agent are self-made in the laboratory.
[0131] Other reagents (such as acids, etc.) are commercially available analytical re...
preparation example 1
[0134] Preparation Example 1: Preparation of (N,N-dihexylamino)methylphosphonic acid bis(2-ethylhexyl)ester
[0135]
[0136] In a three-necked flask equipped with a mechanical stirrer, a water separator, and a condensation reflux device, add diethylhexyl phosphite (1mol), paraformaldehyde (M=30, 1.05mol), di-n-hexylamine (1.05mol) , toluene (700ml) and p-toluenesulfonic acid (2g), heated to reflux at 130°C, and the water generated by the reaction was separated. When anhydrous is produced, react for another 2 hours. 10 g of potassium carbonate was added to the reaction mixture, and heating to reflux was continued for 15 min. The reaction mixture was filtered to remove excess potassium carbonate in the reaction, washed three times with distilled water, and the toluene was removed by rotary evaporation to obtain the target product.
[0137] 1 H NMR (400MHz, CDCl 3 ,ppm): δ1.74-0.86[m,(CH 3 ) 6 ,(CH 2 ) 16 ,(CH) 2 ],2.63[m,(CH 2 )],2.95[t,CH 2 , J=8Hz], 3.96[m, (CH ...
preparation example 2
[0138] Preparation Example 2: Preparation of Dioctyl (N,N-Diisobutylamino)methylphosphonate
[0139]
[0140] Except that di-n-octyl phosphite was used to replace diethylhexyl phosphite and diisobutylamine was used to replace di-n-hexylamine, the target product was prepared by the same process as in Preparation Example 1.
[0141] 1 H NMR (400MHz, CDCl 3 ,ppm): δ1.71-0.89[m,(CH 3 ) 6 ,(CH 2 ) 12 ],2.05[m,(CH) 2 ],2.31[m,(CH 2 ) 2 ],2.95[m,(CH 2 )],4.02[m,(CH 2 ) 2 ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com