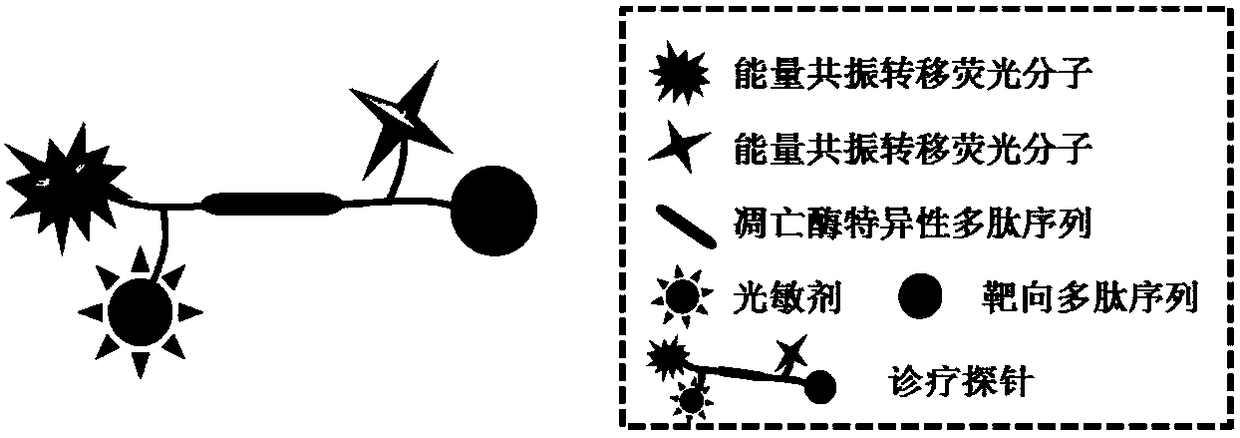
A fluorescent probe for tumor-targeted diagnosis and treatment
A tumor targeting and probe technology, applied in the field of tumor targeted diagnosis and treatment fluorescent probes, can solve problems such as inability to feedback and monitor, and achieve the effects of improving solubility, simple purification process and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] [Example 1] Synthesis of fluorescent probes for tumor-targeted diagnosis and treatment
[0049] Protoporphyrin-lysine (fluorescein)-serine-aspartic acid-glutamic acid-valine-aspartic acid-serine-lysine (dimethylaminoazobenzene)-arginine- Glycine-aspartic acid, PpIX-K(FAM)SDEVDSK(Dabcyl)RGD) synthesis at room temperature:
[0050] (1) Add 0.5 g of ammonia resin (0.525 mmol / g) to a reactor containing 10 mL of double-distilled N,N-dimethylformamide, and wait until the ammonia resin is dissolved in N,N-dimethylformamide N,N-dimethylformamide was extracted after swelling at room temperature for 2h.
[0051] (2) Add 20% (V / V) piperidine / N,N-dimethylformamide (i.e. the volume ratio of piperidine to N,N-dimethylformamide is 2:8) solution 10mL into the reactor After reacting at room temperature for 15 minutes, the solvent was removed; piperidine / N,N-dimethylformamide solution was repeatedly added for reaction to cut off the FMOC protecting group. After the reaction was complet...
Embodiment 2
[0063] [Example 2] Response detection of tumor-targeted diagnosis and treatment fluorescent probes to caspase-3
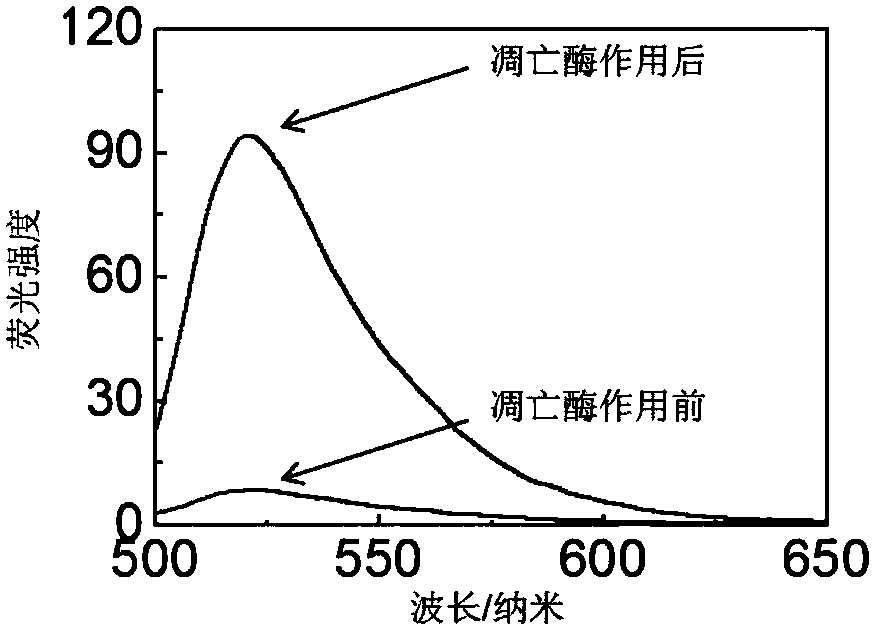
[0064] Dissolve the probe in HEPES buffer solution to make a working solution of 1 μmol / L. Apoptase-3 (1 U) was added to the probe-containing buffer solution, and the working solution was diluted with the buffer solution to a final concentration of 0.5 μmol / L. The fluorescence intensity of the luciferin in the solution was detected by a fluorescence spectrometer (LS55 fluorescence spectrophotometer, Perkin-Elmer) when the caspase-3 was just added and 11 hours after the caspase-3 was added. The excitation wavelength of fluorescein is 465 nm.
[0065] The result is as figure 2 As shown, the luciferin fluorescence intensity of the probe in the solution was weak when caspase-3 was just added, and after 11 hours of action with caspase-3, the luciferin intensity of the probe was about 11 times higher at 520 nm. enhanced. Therefore, it is proved that the fluorescence...
Embodiment 3
[0066] [Example 3] Specific detection of tumor-targeted diagnosis and treatment fluorescent probes responding to caspase-3
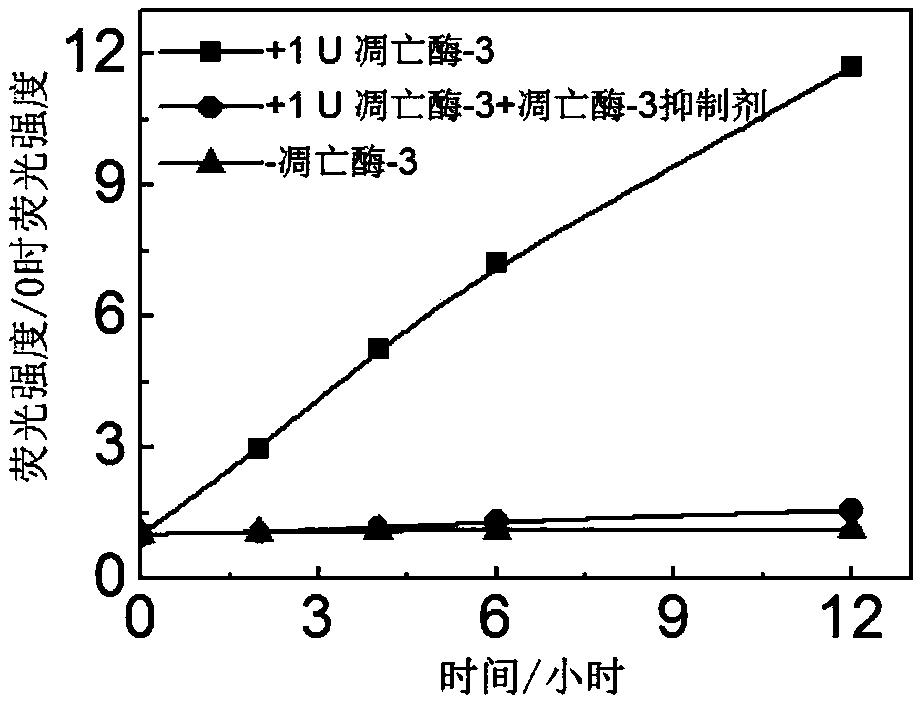
[0067] Apoptase-3 (1U) was incubated with a commercialized caspase-3 specific inhibitor (Ac-DEVD-CHO, 50 μmol / L) at 37°C for 2 hours. The probe solution was prepared as a 1 μmol / L working solution in HEPES buffer solution. Add apoptase-3 (1U) to the buffer solution containing the probe, apoptase-3 (1U) incubated with apoptase inhibitor, and dilute the probe concentration to a final concentration of 0.5 μmol / Lift. Use a fluorescence spectrometer to record how the fluorescence of the probe solution without adding captase-3, adding caspase-3, and adding caspase-3 and inhibitor changes with time. Excitation wavelength of fluorescein: 465 nm; emission wavelength of collected fluorescein: 520 nm.
[0068] The result is as image 3 As shown, without the action of apoptase-3, the fluorescein fluorescence intensity of the probe hardly recovers, while under t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com