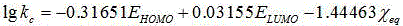Method for predicting elimination rate coefficient of gas state sulfur compound on low-temperature hydrolysis condition
A technology for eliminating rate constants and compounds, applied in electrical digital data processing, special data processing applications, instruments, etc., to achieve good fitting ability, clear application fields, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
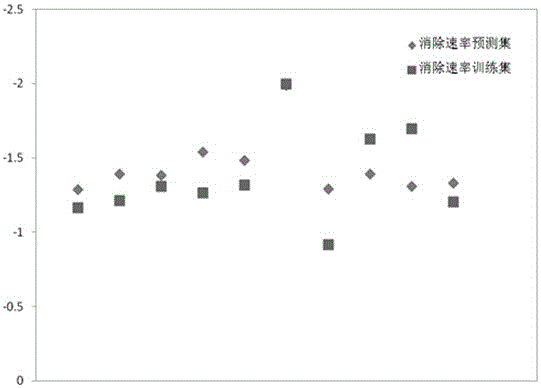
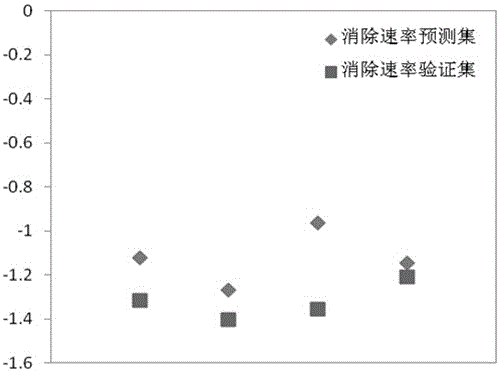
[0027] Example 1: Prediction of gaseous sulfur compounds CS 2 Method for eliminating rate constants under low temperature hydrolysis conditions
[0028] First check the literature to get CS 2 The molecular structure information (S=C=S), and then use the quantum chemical software Gaussian09 to optimize the molecular structure to obtain CS 2 The optimal configuration of the structure (the bond length of C=S is 1.59058?, ∠S-C-S=180°), and then obtain the descriptor required by the model: the highest occupied orbital energy (E HOMO ) is -7.80803eV, the lowest unoccupied orbital energy (E LUMO ) is -2.32288eV, and the molecular balance electronegativity is 2.5699; , where (N is the total number of atoms in the molecule, is the electronegativity of the atom, is the atomic number of a certain atom in the molecule), the predicted value of the elimination rate constant is -1.3145, while the experimental value found is -1.1208, and the error is only 0.1937, which is very consiste...
Embodiment 2
[0029] Example 2: Method for predicting the elimination rate constant of gaseous sulfur-containing compound COS under low temperature hydrolysis conditions
[0030] First, check the literature to obtain the molecular structure information of COS (O=C=S), and then use the quantum chemical software Gaussian09 to optimize the molecular structure to obtain the optimal configuration of the COS structure (the bond length of O=C is 1.17652?, S= The bond length of C is 1.60297?, ∠O-C-S=180°), and then obtain the descriptor required by the model: the highest occupied orbital energy (E HOMO ) is -8.49293eV, the lowest unoccupied orbital energy (E LUMO ) is -1.4076eV, molecular balance electronegativity was 2.8026; finally passed ,in (N is the total number of atoms in the molecule, is the electronegativity of the atom, is the atomic number of an atom in the molecule), the predicted value of the elimination rate constant is -1.40512, while the experimental value found is -1.2699...
Embodiment 3
[0031] Embodiment 3: method for predicting the elimination rate constant of gaseous methyl sulfide under low temperature hydrolysis conditions
[0032] First check the literature to obtain the molecular structure information of methyl sulfide ((H 3 C-S-CH 3 )), and then use the quantum chemical software Gaussian09 to optimize the molecular structure to obtain the optimal configuration of the methyl sulfide structure (symmetric configuration, the bond length of C-S is 1.89074?, ∠C-S-C=98.74994°), and then obtain the required Descriptor of: highest occupied orbital energy (E HOMO ) is -6.19045eV, the lowest unoccupied orbital energy (E LUMO ) is 0.5867eV, molecular balance electronegativity was 2.3082; finally passed ,in (N is the total number of atoms in the molecule, is the electronegativity of the atom, is the atomic number of an atom in the molecule), the predicted value of the elimination rate constant is -1.35671, while the experimental value found is -0.9641, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com