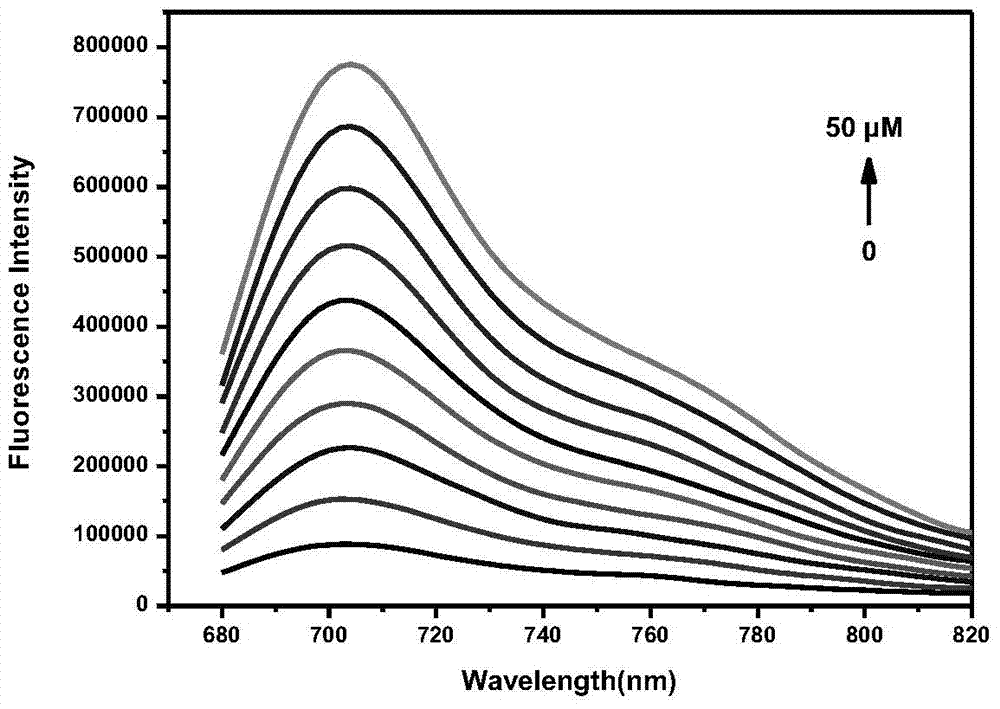
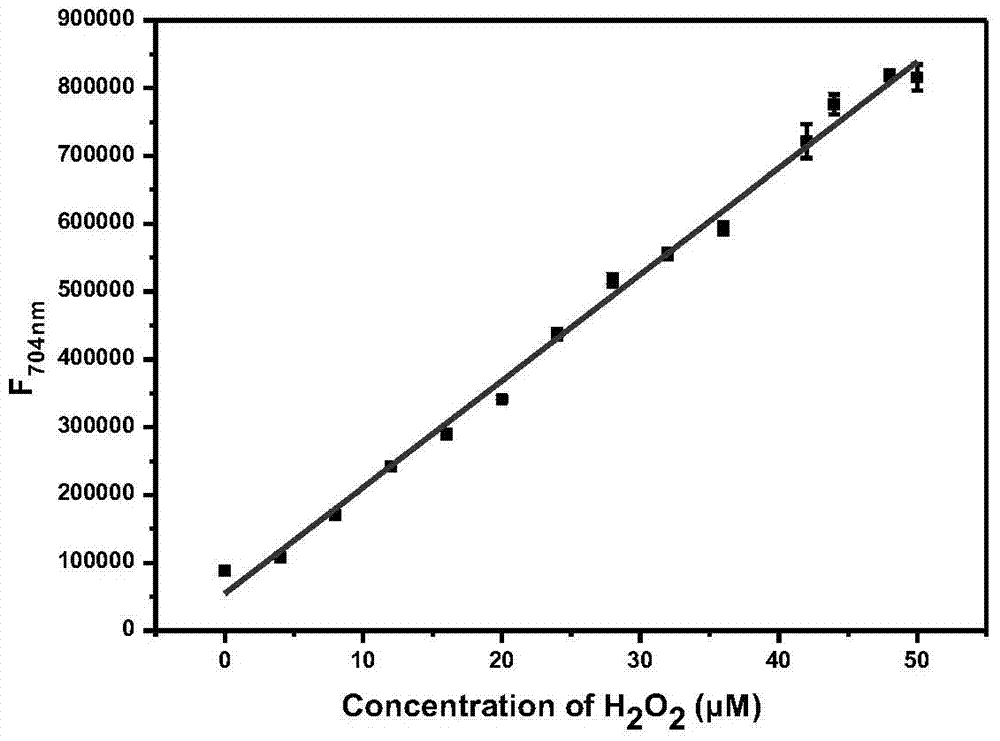
A fluorescent probe for detecting hydrogen peroxide and its preparation method and application
A hydrogen peroxide and fluorescent probe technology, which is applied in the field of fluorescent probes, can solve the problems that the selectivity of hydrogen peroxide needs to be improved, the selectivity of the probe is not good, and the in vivo imaging cannot be used, so as to achieve novel responsive groups, The synthesis method is simple and the effect of little interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] (1) 3-Aminophenol (436mg), (Boc) 2 O (1.04g) was dissolved in absolute ethanol (15mL), refluxed at 80°C for 5h, and spin-dried to obtain a white solid (Ph-Boc).
[0070] Mass Spectrometry Characterization:
[0071] HRMS (ESI - ): m / z calcd for[M-H]=208.0968,[2M-H]=417.2020, Found208.1114, 417.2035.
[0072] (2) Dissolve Cy.7.Cl (200mg), sodium hydride (19.2mg) and Ph-Boc (163mg) obtained in step (1) in DMF (3mL), react at 25°C for 2h, spin dry, and use The chromatographic column was separated (eluent V 石油醚 :V 二氯甲烷 :V 无水乙醇 =25:25:1), spin-dried to obtain a green solid (Cy-Ph-Boc).
[0073] Mass Spectrometry Characterization:
[0074] HRMS (ESI - ):m / z calcd for[M-I] + =684.4159, Found684.4198.
[0075] (3) Cy-Ph-Boc (200mg) was dissolved in dichloromethane (3mL), ice-bathed for 1h, then trifluoroacetic acid (1mL) was added dropwise, and reacted at 25°C for 6h. Saturated sodium bicarbonate aqueous solution and dichloromethane extraction, the organic phase was d...
Embodiment 2
[0090] (1) 3-Aminophenol (2.18g), (Boc) 2 O (5.2g) was dissolved in absolute ethanol (75mL), refluxed at 80°C for 5h, and spin-dried to obtain a white solid (Ph-Boc).
[0091] Mass Spectrometry Characterization:
[0092] HRMS (ESI - ): m / z calcd for[M-H]=208.0968,[2M-H]=417.2020, Found208.1114, 417.2035.
[0093] (2) Dissolve Cy.7.Cl (0.8g), sodium hydride (76.8mg) and Ph-Boc (0.625g) obtained in step (1) in DMF (12mL), react at 25°C for 2h, spin dry , separated by chromatographic column (eluent V 石油醚 :V 二氯甲烷 :V 无水乙醇 =25:25:1), spin-dried to obtain a green solid (Cy-Ph-Boc).
[0094] Mass Spectrometry Characterization:
[0095] HRMS (ESI - ):m / z calcd for[M-I] + =684.4159, Found684.4198.
[0096] (3) Cy-Ph-Boc (0.450 mg) was dissolved in dichloromethane (6.75 mL), ice-bathed for 1 h, then trifluoroacetic acid (2.25 mL) was added dropwise, and reacted at 25° C. for 6 h. Saturated sodium bicarbonate aqueous solution and dichloromethane extraction, the organic phase wa...
Embodiment 3
[0109] This example is the same as Example 1, except that 4-nitrophenylglyoxylic acid is changed to 4-cyanophenylglyoxylic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com