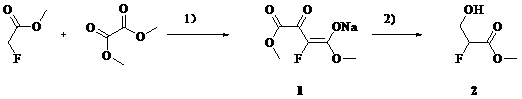
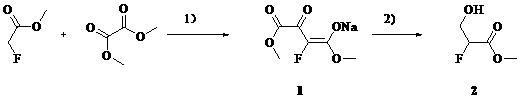
Synthetic method of methyl 2-fluoro-3-hydroxypropionate
A kind of technology of methyl hydroxypropionate and synthesis method, which is applied in the synthesis field of methyl 2-fluoro-3-chloropropionate, and can solve the problem that there is no good method for preparing methyl 2-fluoro-3-hydroxypropionate and other problems, to achieve the effect of low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment one: the synthesis of sodium enolate intermediate, methyl tert-butyl ether is used as solvent
[0019] Add methyl tert-butyl ether (1L), methyl fluoroacetate (460 g), and dimethyl oxalate (620 g) into a 5 L three-necked flask at 20 – 30 o C and stirred for 10 min. NaOMe / MeOH (27% by weight) solution (1050mL) was added dropwise, and the temperature was controlled at 20-30 o c. The color of the solution changed from colorless to yellow immediately. The dropwise addition is complete. Warm 20 – 30 o C reacted for 4 h. After filtration, the filter cake was slurried with methyl tert-butyl ether (500 mL), and suction filtered. Obtained 1 (840 g, 84 %) as a pale yellow solid.
[0020] Its reaction formula is:
[0021]
Embodiment 2
[0022] Embodiment two: the synthesis of sodium enolate intermediate, tetrahydrofuran is made solvent
[0023] Add tetrahydrofuran (1L), methyl fluoroacetate (460 g), and dimethyl oxalate (620 g) into a 5 L three-neck flask at 20 – 30 o C and stirred for 10 min. NaOMe / MeOH (27% by weight) solution (1050 mL) was added dropwise, and the temperature was controlled at 20-30 o c. The color of the solution changed from colorless to yellow immediately. The dropwise addition is complete. Warm 20 – 30 o C reacted for 4h. After filtration, the filter cake was slurried with tetrahydrofuran (500 mL), and suction filtered. Obtained 1 (870 g, 87 %) as a pale yellow solid.
Embodiment 3
[0024] Example Three: Methyl 2-fluoro-3-hydroxypropionate, N,N-dimethylformamide as solvent
[0025] Throw in 5.6L of N,N-dimethylformamide, and cool down to 0 in an ice-water bath o C, throw 700 g enol sodium salt and Na 2 CO 3 360 g of the mixture, at the same time start rapid (within 1 hour) dropwise addition of 37% HCHO aq 263 mL, the addition is complete, 0 - 10 o C, heat preservation reaction for 2 h, add 5L×3EA to extract three times, combine the organic phase, Na 2 SO 4 Dried, filtered, and the filtrate was evaporated to dryness to give 423.4 g, distilled, collected in 100 - 110 o Fraction between C. Yield 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com