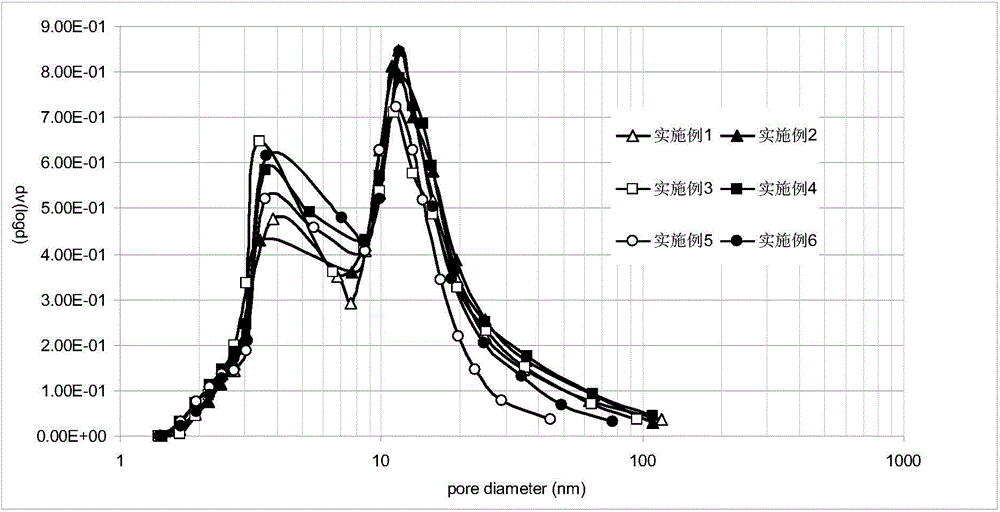
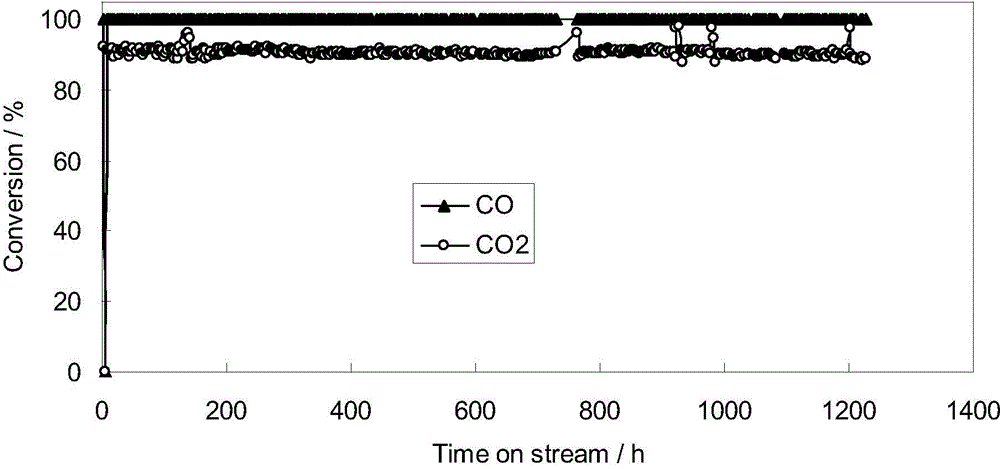
Composite oxide carrier with bimodal mesopores, as well as preparation method and application of composite oxide carrier
A technology of composite oxides and oxides, which is applied in the direction of catalyst carriers, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., which can solve the problem that the carrier’s mechanical strength and long-term running stability need to be improved, and the carrier Structural stability is not obvious, it is not easy to scale up industrial production, etc., to achieve good low-temperature catalytic activity and long-term running stability, good long-term running stability, and suitable for scale-up of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1-1) Weigh the Al of 100 mesh sieves 2 o 3 ·3H 2 O powder 6.5g, Al 2 o 3 ·H 2 O powder 13.0g, γ-Al 2 o 3 Powder 47.5g, ZrO 2 Mix 10.0 g of powder, 0.75 g of hydroxypropyl cellulose and 0.75 g of 425 cement in a dry mixer for 60 minutes to obtain mixed powder A for use.
[0048] (1-2) Weigh La(NO 3 ) 3 ·6H 2 O4.0g was dissolved in deionized water, and the volume was adjusted to 40ml, which was marked as solution B.
[0049] (1-3) Move the mixed powder A in step (1-1) into the wet mixer, and continuously spray the solution B obtained in step (1-2) into the mixed powder in the form of mist while the material powder is rotating. On the surface of powder A, after spraying all the mixed liquid B, continue wet mixing for 60 minutes to obtain mixed wet material C.
[0050] (1-4) Granulate the mixed wet material C obtained in step (1-3), dry at 120°C for 6 hours, and use a tablet machine to make cylindrical granules of Φ3×5mm after cooling.
[0051] (1-5) The molde...
Embodiment 2
[0055] (2-1) Weigh the Al of 100 mesh sieves 2 o 3 ·3H 2 O powder 12.3g, Al 2 o 3 ·H 2 O powder 8.0g, γ-Al 2 o 3 Mix 20.3g of powder, 1.0g of hydroxypropyl cellulose and 1.0g of 425 cement in a dry mixer for 60 minutes to obtain mixed powder A for later use.
[0056] (2-2) Weigh La(NO 3 ) 3 ·6H 2 O20.0g and Co(NO 3 ) 2 ·6H 2 O32.0g was dissolved in deionized water, and the volume was adjusted to 60ml, which was marked as solution B.
[0057] (2-3) Move the mixed powder A in the step (2-1) into the wet mixer, and continuously spray the solution B obtained in the step (2-2) into the mixing machine in the form of mist while the material powder is rotating. On the surface of powder A, after spraying all the mixed liquid B, continue wet mixing for 60 minutes to obtain mixed wet material C.
[0058] (2-4) The mixed wet material C obtained in the step (2-3) is made into Φ3×5mm cylindrical particles with an extruder.
[0059] (2-5) Dry the molded carrier at 120°C for 6 ...
Embodiment 3
[0063] (3-1) Weigh the Al of 100 mesh sieves 2 o 3 ·3H 2 O powder 10.2g, Al 2 o 3 ·H 2 O powder 7.6g, γ-Al 2 o 3 Mix 12.0g of powder, 1.0g of turnip powder and 0.5g of 425 cement in a dry mixer for 40 minutes to obtain mixed powder A for later use.
[0064] (3-2) Weigh Zr(NO 3 ) 4 ·3H 2 O40.0g and Co(NO 3 ) 2 ·6H 2 O16.7g was dissolved in deionized water, and the volume was adjusted to 60ml, which was marked as solution B.
[0065] (3-3) Move the mixed powder A in the step (3-1) into the wet mixer, and continuously spray the solution B obtained in the step (3-2) into the mixing machine in the form of a mist while the material powder is rotating. On the surface of powder A, after spraying all the mixed liquid B, continue wet mixing for 60 minutes to obtain mixed wet material C.
[0066] (3-4) The mixed wet material C obtained in the step (3-3) is made into Φ3×5mm cylindrical particles with an extruder.
[0067] (3-5) Dry the molded carrier at 120°C for 6 hours, h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com