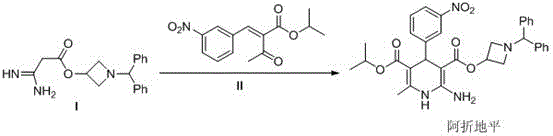
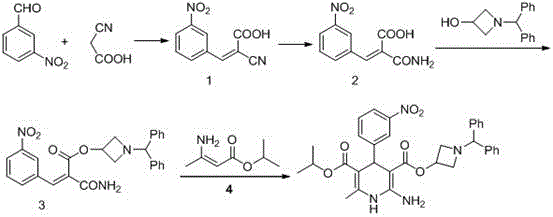
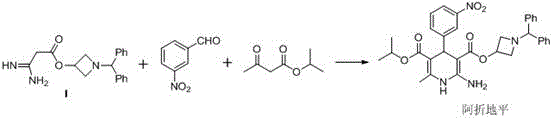
Preparation method of azelnidipine
A technology of azelnidipine and its compound, which is applied in the field of preparation of azelnidipine, can solve the problems of compound 1 production limitation, etc., and achieve the effects of easy separation, simple operation, and avoiding synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 5L reaction flask, add 2L of absolute ethanol, 6g of glacial acetic acid, 8.5g of piperidine and stir for 0.5h at 20~30°C, then add 170g of cyanoacetic acid, 302g of m-nitrobenzaldehyde, and react at 20~30°C for 24h ( TLC central control). Suction filtration after completion of the reaction, the filter cake was rinsed with 200ml of absolute ethanol, and the dried product was 405g, with a yield of 93% and a purity of 98.5%.
[0030] Synthesis of Compound 2
[0031] Add 400g of compound 1, 1.5kg of 30% KOH aqueous solution, and 2L of ethanol into a 5L reaction flask, heat to reflux for 6 hours, cool down to 20~30°C, adjust the pH to 1~2 with industrial hydrochloric acid, a large amount of solids are precipitated, pump Filter, dry product 381g after a small amount of ethanol rinse, yield 88%, purity 99%.
[0032] Synthesis of Compound 3
[0033] In the reaction flask, add 200g of compound 2, 3.5L of tetrahydrofuran and 200g of 1-benzhydryl-3-azetidinol, stir to coo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com