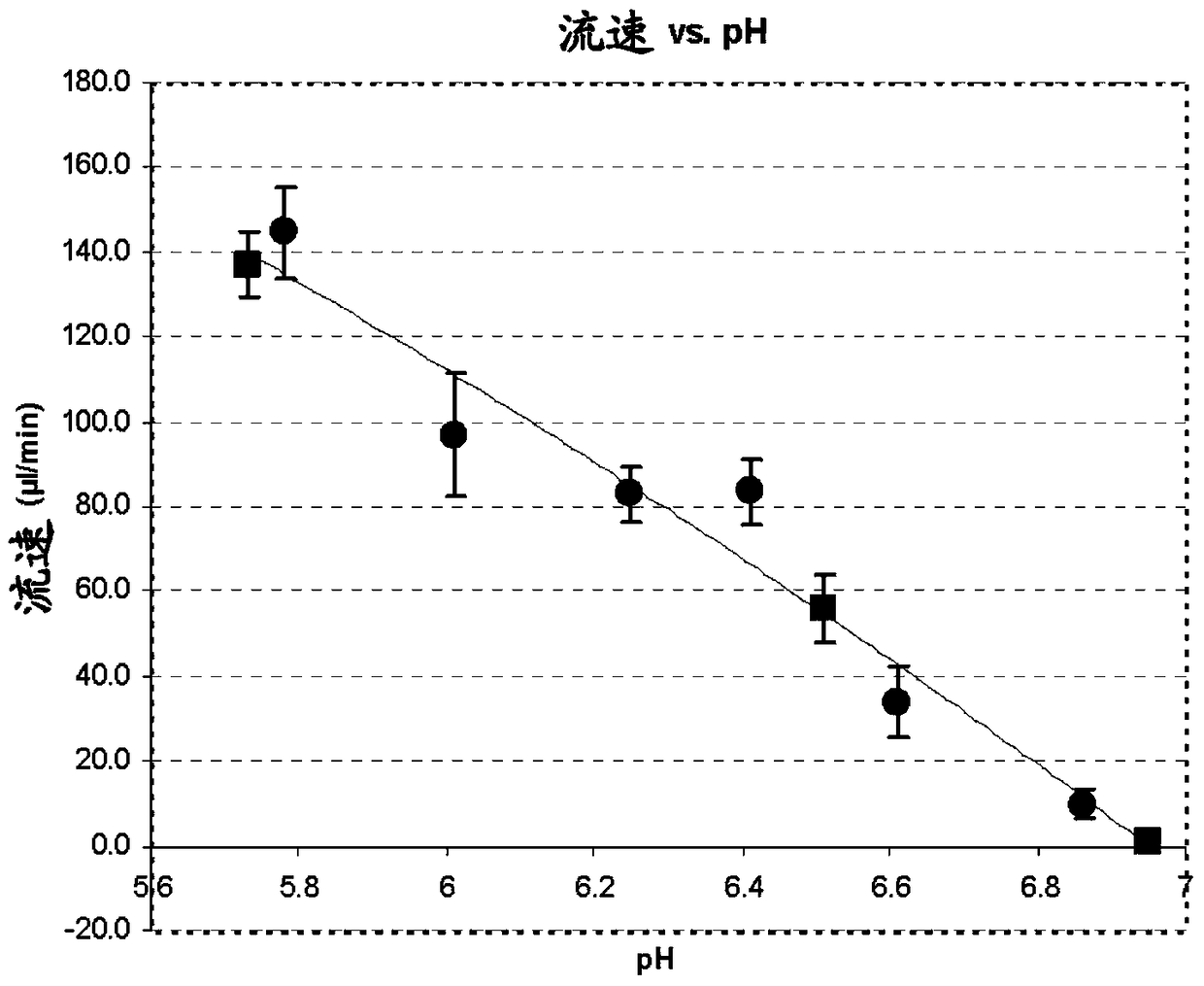
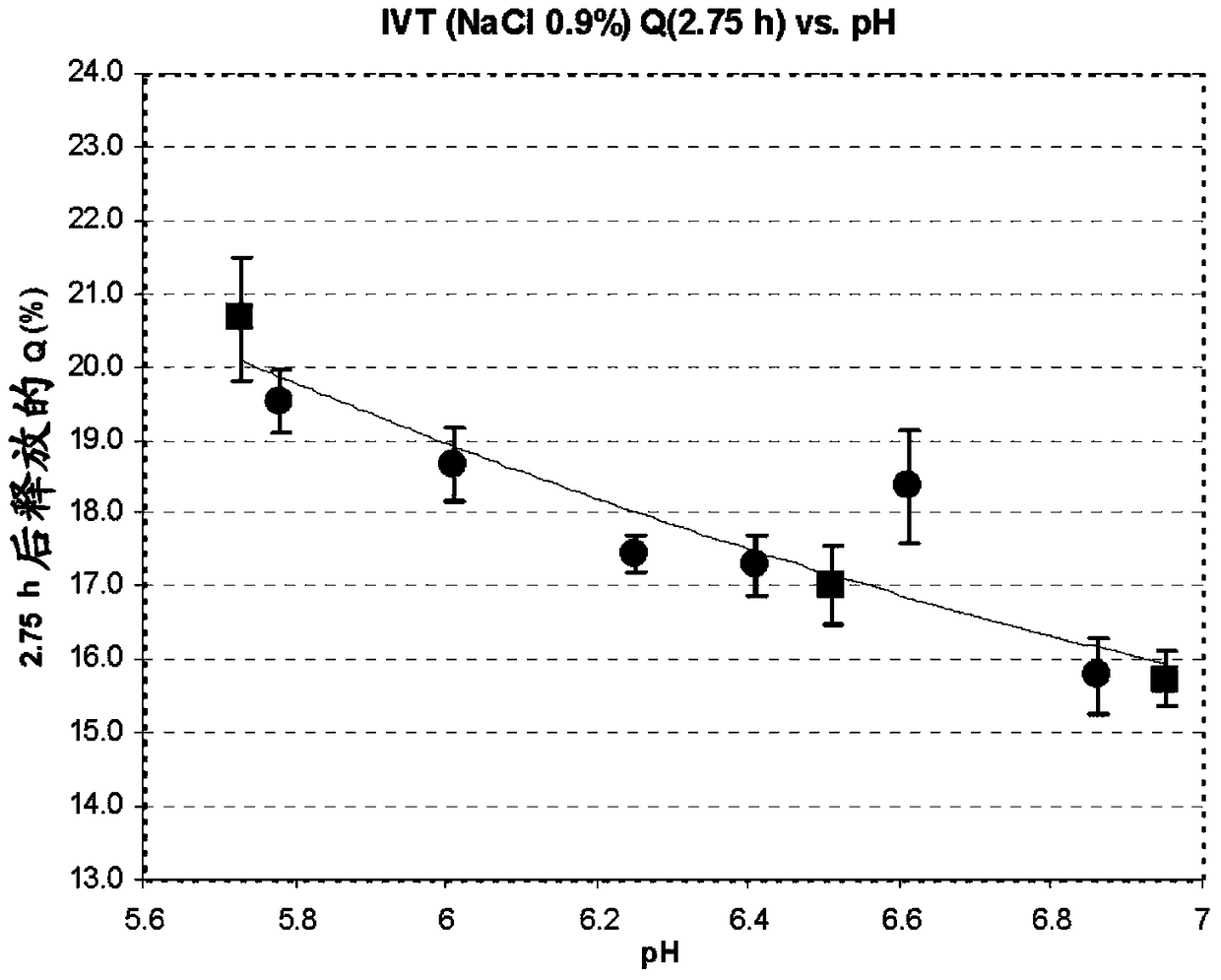
Preparation method of pharmaceutical composition for delayed release of somatostatin analogs
A technology for delayed release and composition, applied in the field of preparation of the pharmaceutical composition of the delayed release somatostatin analogue lanreotide, and the preparation of the pharmaceutical composition, which can solve the problems of not adding acid publicly and achieve the effect of improving convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 10
[0120] Example 10: Preferred method of preparing compositions of the invention
[0121] Pre-lyophilized cells were prepared by dissolving 25 g / l of pure lanreotide [BIM-23014] in 15% aqueous acetic acid. The cells were placed in empty metal trays, cooled from room temperature to 2°C over 10 minutes and then held at this temperature for 3 hours. The cooled mixture was then frozen to -40°C within 10 minutes and then held at this temperature for 2.75 hours. A vacuum of 20 μbar was applied and this vacuum was maintained while the mixture was heated from -40°C to 25°C over 20 hours, then heated to 35°C and held for 16.75 hours.
[0122] The water for injection is acidified with glacial acetic acid in an amount sufficient to provide anhydrous acetate with a final content of 9.6%-10%. The acidified water and lanreotide acetate were then mixed as follows to obtain a concentration of 24.6% pure lanreotide and 9.6%-10% anhydrous acetate: weigh lanreotide acetate and water, place In t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com