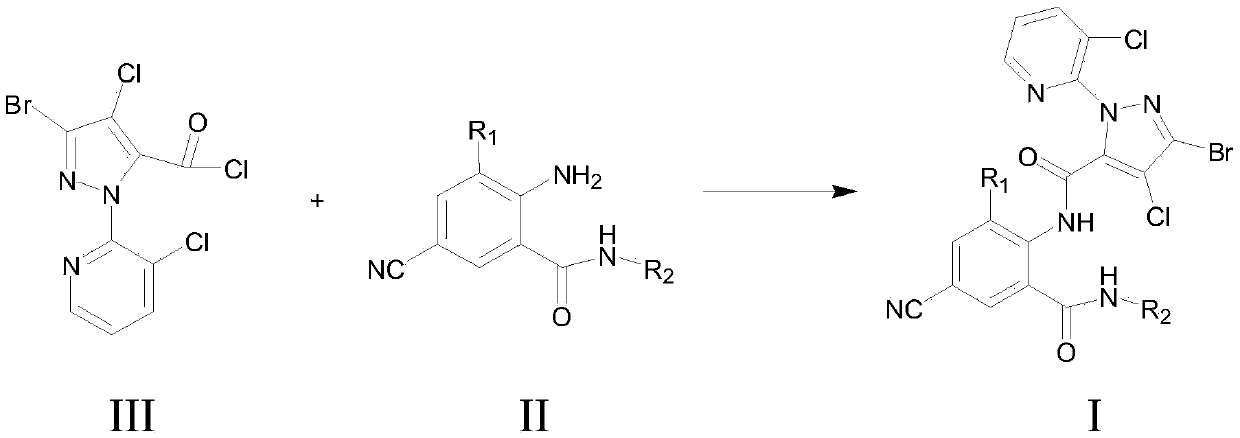
Cyan dihalogenation pyrazol amide series compound and preparation method and application thereof
A technology for cyanodihalogenated pyrazole amides and compounds is applied in the field of cyanodihalogenated pyrazole amides and their preparation, which can solve problems such as unpublished application performance, and achieve high insecticidal activity, insecticidal activity and the like. The effect of broad spectrum and strong systemic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 compound 1,
[0028] (1) Synthesis of 1-(3-chloropyridin-2-yl)-3-bromo-4-chloro-1H-pyrazole-5-carboxylic acid ethyl ester
[0029]
[0030] Add 33.25g (0.1mol) 1-(3-chloropyridin-2-yl)-3-bromo-4,5-dihydro-1-H pyrazole-5-carboxylic acid ethyl ester and 100ml acetonitrile to the 250ml reaction flask , adding 27g (0.2mol) of sulfonyl chloride under stirring, refluxed for 4h, thin-layer chromatography TLC detected that the raw material disappeared, and the solvent was distilled off to obtain 30g of yellow oily liquid with a yield of 82%.
[0031] Among them, 1-(3-chloropyridin-2-yl)-3-bromo-4,5-dihydro-1H pyrazole-5-carboxylic acid ethyl ester references (Wang Yanjun, Zhang Dayong, Wu Xiaoming, Chlorantraniliprole Synthesis of [J]. Pesticides, 2010, (03): 170-174).
[0032] (2) Synthesis of 1-(3-chloropyridin-2-yl)-3-bromo-4-chloro-1H-pyrazole-5-carboxylic acid
[0033]
[0034] Add 36.6g (0.1mol) 1-(3-chloropyridin-2-yl)-3-bromo-4-c...
Embodiment 2
[0055] The synthesis of embodiment 2 compound 2
[0056] (1) Synthesis of 2-amino-5-cyano-N-ethyl-3-methylbenzamide
[0057]
[0058] Take 6-cyano-8-methyl-1H-benzo[d][1,3]oxazine-2,4-dione 2.0g (0.01mol), acetonitrile 20ml, triethylamine 2g (0.02mol) In a 50ml single-necked bottle equipped with a stirring magnet, add 4g (0.02mol) of ethylamine aqueous solution dropwise under stirring, stir at room temperature for 2h until the reaction is clear, distill off the solvent, extract the reaction with ethyl acetate three times, combine the organic phases and use It was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed to obtain 1.5 g of a yellow solid with a yield of 62%.
[0059] (2) Synthesis of Compound 2
[0060]
[0061] Add 2.13g (0.01mol) 2-amino-5-cyano-N-ethyl-3-methylbenzamide to a 100ml reaction flask, 10ml acetonitrile, drop 3.92g (0.011mol) 1-(3-chloro A mixed solution of pyridin-2-yl)-3-bromo-4-chloro-1H-pyrazole-5-f...
Embodiment 3
[0063] The synthesis of embodiment 3 compound 4
[0064] (1) Synthesis of 2-amino-5-iodo-3-chlorobenzoic acid
[0065]
[0066] Put 5.2g (0.033mol) of 2-amino-3-chlorobenzoic acid in a 100ml reaction flask, add 30ml of DMF, add NIS iodide reagent (7.8g, 0.035mol) under stirring at room temperature, raise the temperature to 80°C for 2h, TLC After monitoring the completion of the reaction, the temperature was lowered to 30° C., the reaction solution was poured into ice water, stirred for 20 min, and filtered to obtain 8.9 g of a gray solid with a yield of 95%.
[0067] (2) Synthesis of 2-amino-5-cyano-3 chlorobenzoic acid
[0068]
[0069] 2-Amino-5-iodo-3-chlorobenzoic acid 4.37g (0.015mol) CuCN1.87g (0.021mol) in a 100ml reaction flask, add 50ml of DMF, the temperature rises to 145°C to react, keep the temperature for 5h, TLC detection After the reaction was complete, most of the solvent was removed under reduced pressure, 50ml of water and 5ml of ethylenediamine were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com