Gene rEg.P29 molecular engineering vaccine resistant to sheep echinococcosis infection and preparation method and application thereof
A gene engineering vaccine, echinococcosis technology, applied in the field of gene rEg.P29 molecular engineering vaccine against sheep echinococcosis infection and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
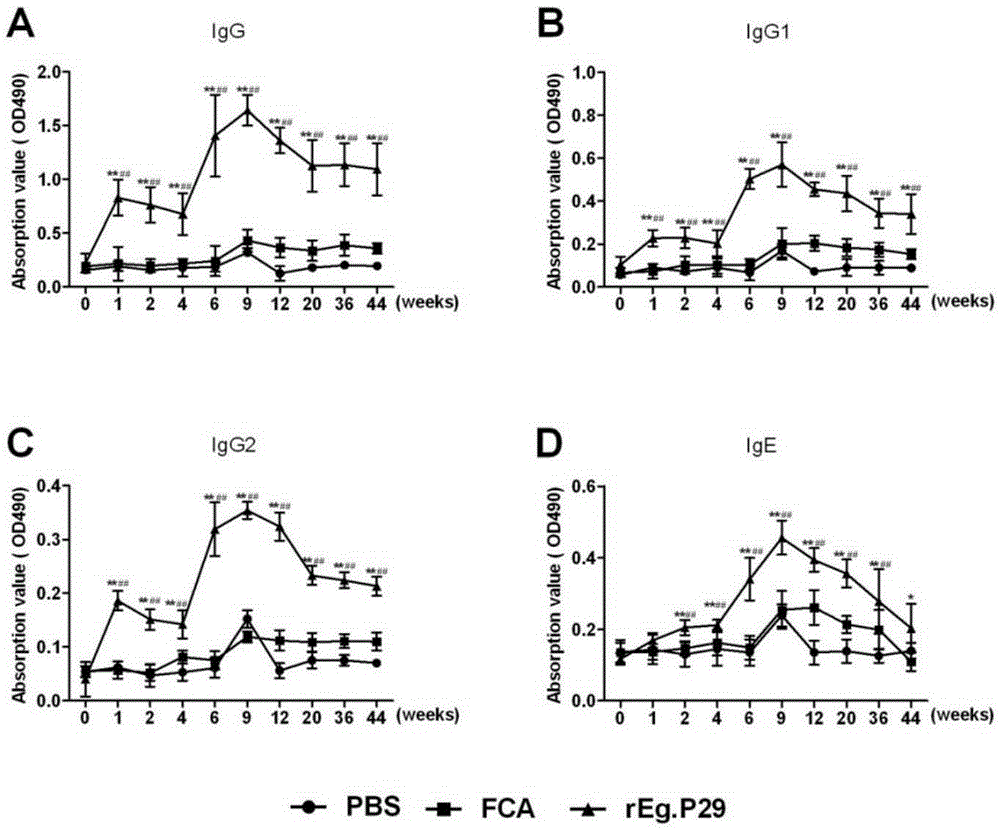
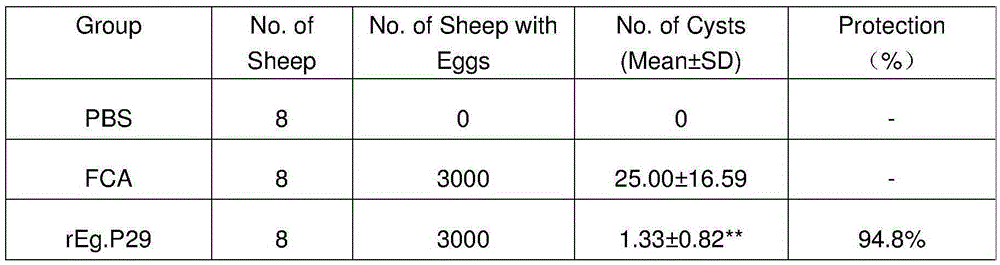
[0016] Vaccine preparation:
[0017] Firstly, RNA was extracted from the Chinese strain of Echinococcus granulosus and the Eg.P29 gene was obtained by RT-PCR. The gene was included in the GenBank database with the sequence number AF078931. Secondly, the prokaryotic expression vector was constructed, and IPTG induced expression in Escherichia coli BL21. The protein rEg.P29 was identified by SDS-PAGE and Western blot, purified by His-tag affinity chromatography, and stored at -20°C.
[0018] Verification of vaccine invention effect:
[0019] 1. Preparation of experimental animals and Echinococcus granulosus eggs
[0020] Male sheep aged 4-6 months, who were negative for sheep serum (ELISA) detected by the crude antigen coating of protocercariae, were randomly divided into 3 groups: rEg.P29 vaccine group, FCA (complete Ford's adjuvant) group, PBS group, 10 rats in each group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com