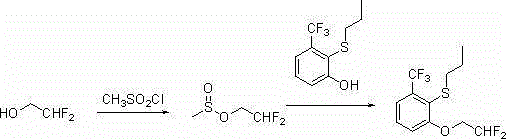
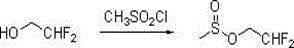2-(2 ', 2'-difluoro-ethoxy)-6-trifluoromethyl-phenylpropyl sulfur ether synthesis process
A technology of trifluoromethylphenylpropyl sulfide and difluoroethoxy, which is applied in the preparation of sulfide, organic chemistry, etc., can solve the problems of high price, difficult purification, and many reaction by-products, etc. Industrial value and prospects, simple separation and purification, high content and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of 2,2-difluoroethyl methanesulfonate:
[0022] Add 41.44g (0.5mol, 99%, 1.0eq) of 2,2-difluoroethanol, 150mL of toluene, and 75.21g of methanesulfonyl chloride (0.65mol, 99%, 1.3eq) into a 500mL four-neck bottle for nitrogen replacement. , the system was a colorless transparent solution. The system was cooled to 0-5°C, and 66.31g (0.65mol, 99%, 1.3eq) of triethylamine was started to be added dropwise to the system, and the temperature was raised to 20-25°C for 1 hour of insulated reaction. Add 200mL of water to the system for extraction and separation, the oil layer was washed with 150mL of water for separation, and the solvent was removed by rotary evaporation under reduced pressure to obtain 79.27g of an orange liquid product with a content of 95.61% and a yield of 94.8%.
[0023] Synthesis of 2-(2',2'-difluoroethoxy)-6-trifluoromethylphenylpropylsulfide
[0024] 24.4g (0.1mol, 96.82%, 1.0eq) of 2-propylmercapto-3-trifluoromethylphenol, 21.7g of 2,2-diflu...
Embodiment 2
[0026] Synthesis of 2,2-difluoroethyl methanesulfonate:
[0027] Add 41.44g (0.5mol, 99%, 1.0eq) of 2,2-difluoroethanol, 150mL of ethyl acetate, and 75.21g of methanesulfonyl chloride (0.65mol, 99%, 1.3 eq), the system was a colorless transparent solution. The system was cooled to 0-5°C, and 66.31g (0.65mol, 99%, 1.3eq) of triethylamine was started to be added dropwise to the system, and the temperature was raised to 20-25°C for 1 hour of insulated reaction. Add 200 mL of water to the system for extraction and separation, wash the oil layer with 150 mL of water for separation, and remove the solvent by rotary evaporation under reduced pressure to obtain 77.9 g of an orange liquid product with a content of 95.6% and a yield of 93.2%.
[0028]Synthesis of 2-(2',2'-difluoroethoxy)-6-trifluoromethylphenylpropyl sulfide:
[0029] 24.4g (0.1mol, 96.82%, 1.0eq) of 2-propylmercapto-3-trifluoromethylphenol, 21.7g of 2,2-difluoroethyl methanesulfonate (0.13mol, 95.6%, 1.3eq), anhydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com