A kind of triptorelin sustained-release microparticles and preparation method thereof
A slow-release microparticle and microparticle technology, which is applied in the direction of diseases, antineoplastic drugs, bulk delivery, etc., can solve the problems of sudden release, poor uniformity of microspheres, low drug loading and encapsulation efficiency, and achieve simple process, Good plasticity and good drug loading effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
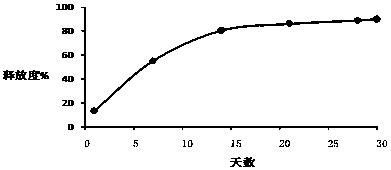
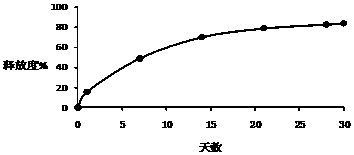
[0026] Example 1-3: Microparticles were prepared according to different types of PLGA, and the release time, drug loading and encapsulation efficiency of the prepared triptorelin microparticles were investigated under the same preparation conditions.
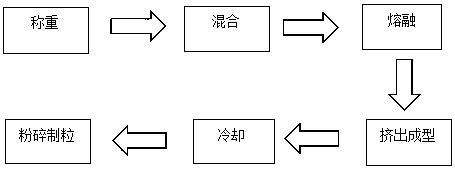
[0027] Weigh 50 mg of triptorelin (4.572%), 1 g of PLGA (95.048%) (see Table 1 for the model of PLGA, different models represent different mass ratios of lactic acid and glycolic acid), 2.1 mg of poloxamer 188 (0.2%) , into the feeding funnel, set the speed of the feeding funnel to 120 rpm, the hot melt extrusion temperature to 80°C, the hot melt extrusion time to 20 minutes, and the crushing and cooling temperature after extrusion to -90°C to collect the crushed particles, Check physical and chemical properties. The test results are shown in Table 1 below.
[0028] Table 1 Microparticles prepared by different types of PLGA
[0029] Example
Embodiment 4-6
[0030] Example 4-6: Microparticles were prepared according to different amounts of PLGA, and the release time, drug loading and encapsulation efficiency of the prepared triptorelin microparticles were investigated under the same preparation conditions.
[0031] Weigh 50 mg of triptorelin, different amounts of PLGA of 75:25 type, poloxamer 188 0.4%, and send them into the feeding funnel, set the feeding funnel speed at 120 rpm, and the hot melt extrusion temperature at 80°C. The hot-melt extrusion time is 20 minutes, the pulverization cooling temperature after extrusion is -90°C, the pulverized particles are collected, and the physical and chemical properties are tested. The test results are shown in Table 2 below.
[0032] Table 2 Microparticles prepared with different ratios of triptorelin and PLGA
[0033] Example
Embodiment 7-15
[0034] Example 7-15: 9 parts of materials were weighed with the prescription ratio of 4.5% triptorelin, 95% PLGA, and 0.5% poloxamer, and prepared by combining the process parameters in the following table 3 respectively. Microparticles were tested for encapsulation efficiency, drug loading, and release time.
[0035] Table 3 Microparticles prepared by different process parameters
[0036]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com