Solid dispersion capsule for treatment of peptic ulcer
A solid dispersion capsule and solid dispersion technology, applied in the digestive system, capsule delivery, medical preparations of non-active ingredients, etc., can solve the problems of high cost, high medicinal taste, large amount of medicinal materials, etc., and achieve improved anti-ulcer activity and pain relief Good effect, the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 pharmaceutical composition of the present invention
[0027] Zushima 3g, Anisopolamine 0.5g, Dried Ginger 9g, Seabuckthorn 10g; after drying, crush to 300 mesh, mix evenly to get raw material medicine powder.
[0028] Take raw drug powder: polyethylene glycol-6000=1:1w / w, mix well, heat until polyethylene glycol-6000 is in a molten state, stir, immediately place in 0°C environment and quench until solidified, crush to obtain Solid dispersion.
[0029] Capsule the solid dispersion to obtain solid dispersion capsules.
Embodiment 2
[0030] The preparation of embodiment 2 pharmaceutical compositions of the present invention
[0031] Zushima 1g, Anisopolamine 0.6g, Dried Ginger 6g, Seabuckthorn 10g; after drying, crush to 400 mesh, mix evenly to get the raw material medicine powder.
[0032] Take raw drug powder: polyethylene glycol-6000=1:3w / w, mix well, heat until polyethylene glycol-6000 is in a molten state, stir, immediately place in 0°C environment and quench until solidified, crush to obtain Solid dispersion.
[0033] Capsule the solid dispersion to obtain solid dispersion capsules.
Embodiment 3
[0034] The preparation of embodiment 3 pharmaceutical composition of the present invention
[0035] Zushima 1g, anisopolamine 0.3g, dried ginger 10g, seabuckthorn 6g; after drying, crush into 500 meshes, and mix well to obtain the raw material medicine powder.
[0036] Take raw drug powder: polyethylene glycol-6000=3:1w / w, mix well, heat until polyethylene glycol-6000 is in a molten state, stir, immediately place in 0°C environment and quench until solidified, pulverize to obtain Solid dispersion.
[0037] Capsule the solid dispersion to obtain solid dispersion capsules.
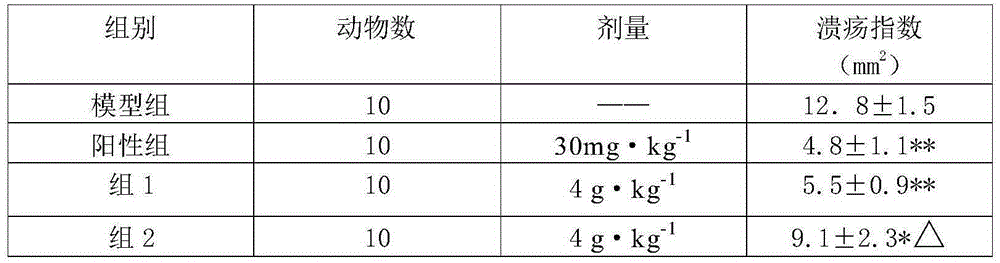
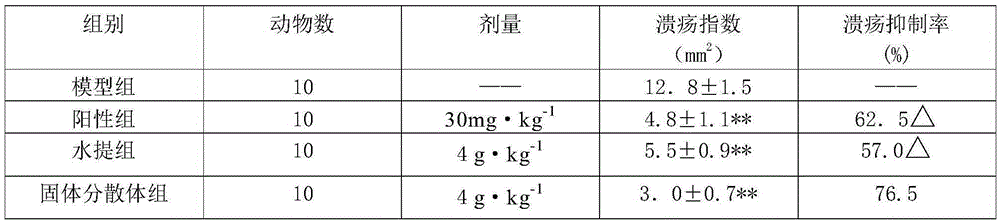
[0038] The beneficial effects of the present invention are further demonstrated through specific test examples below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com