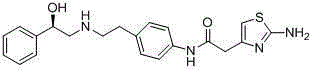
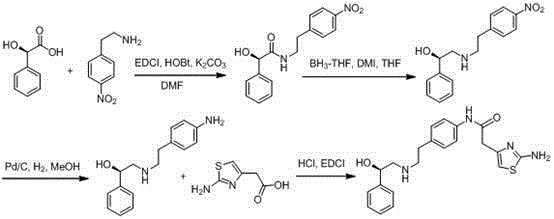
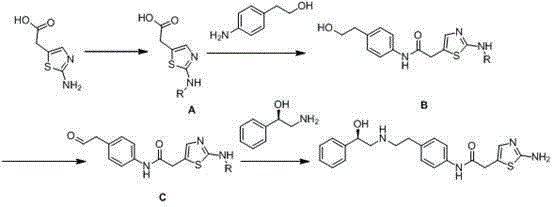
Mirabegron preparation method
A technology of phenyl and amino, which is applied in the field of preparation of miregron, can solve the problems of high price and unsuitability for industrial production, and achieve the effect of simple and easy operation, good repeatability, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] In a 500mL reaction flask, add 20g (R)-1-phenyl-1,2-ethanediol (0.145mol) and 25g (0.29mol) piperidine into 200mL dichloromethane, at 0°C, Slowly add 100mL dichloromethane solution with 30g p-toluenesulfonyl chloride (0.16mol) dropwise. After the dropwise addition, raise to room temperature and react for 1h. TLC monitors the complete reaction of the raw materials. Add a certain amount of water to wash the reaction solution and separate the organic phase. , the aqueous phase was extracted with dichloromethane, the organic phases were combined, and the organic phase was evaporated to obtain 35 g of (R)-1-phenyl-1,2-hydroxy-p-toluenesulfonate.
Embodiment 2
[0035]
[0036] In a 500mL reaction flask, add 20g (R)-1-phenyl-1,2-ethanediol (0.145mol) and 29g (0.29mol) triethylamine into 200mL dichloromethane, at 0°C , slowly add dichloromethane solution with 30g of p-toluenesulfonyl chloride (0.16mol) dropwise, after the dropwise addition, rise to room temperature and react for 4h, TLC monitors the complete reaction of raw materials, adjust the pH of the reaction solution to neutral with glacial acetic acid, add Wash the reaction solution with a certain amount of water to separate the organic phase, then extract the water phase with dichloromethane, combine the organic phases, and evaporate the organic phase to obtain 33g (R)-1-phenyl-1,2-hydroxy-p-toluenesulfonate esters.
Embodiment 3
[0038]
[0039] In a 500mL reaction flask, add 20g (R)-1-phenyl-1,2-ethanediol (0.145mol) and 25g (0.29mol) piperidine into 200mL dichloromethane, at 0°C, Slowly add 100 mL of dichloromethane solution containing 18 g of methanesulfonyl chloride (0.16 mol) dropwise. After the dropwise addition, rise to room temperature and react for 1 h. TLC monitors that the raw materials have reacted completely. Add a certain amount of water to wash the reaction solution and separate the organic phase. The aqueous phase was extracted with dichloromethane again, the organic phases were combined, and the organic phase was distilled off to obtain 27 g of (R)-1-phenyl-1,2-hydroxy-methylsulfonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com