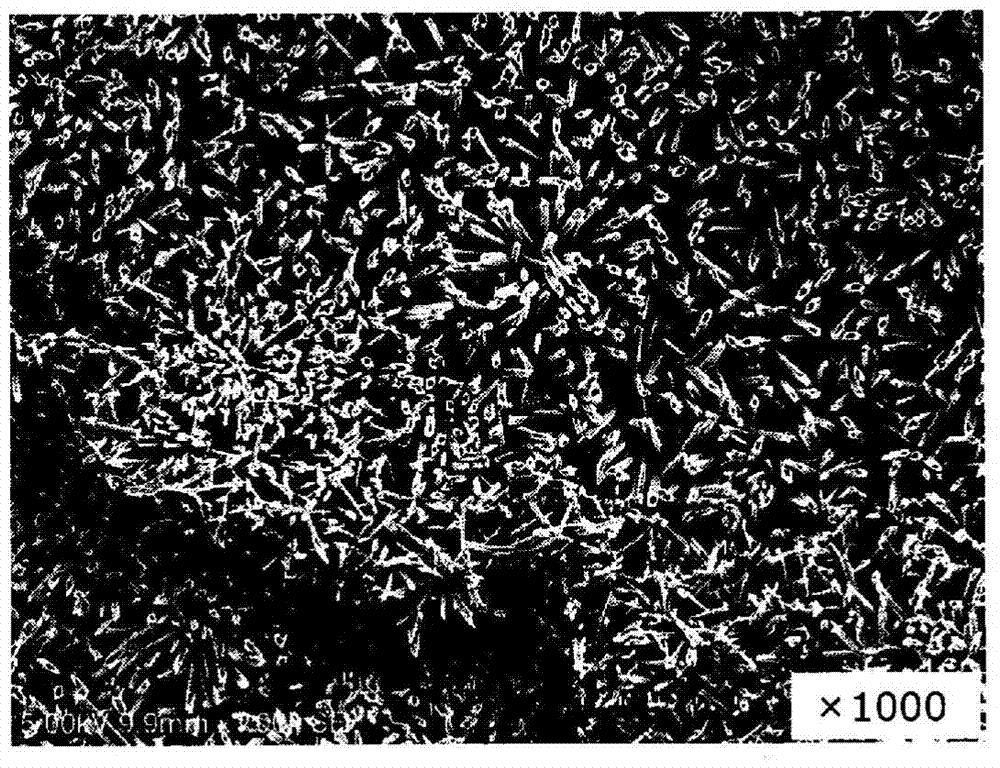
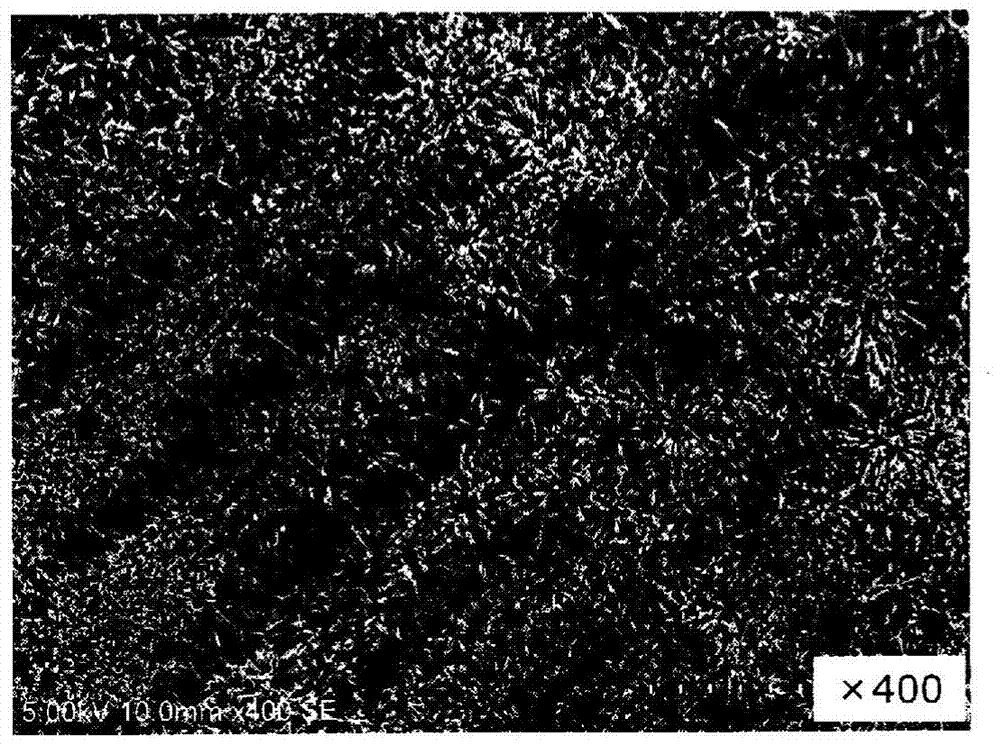
drug coating
A drug coating and drug technology, applied in coatings, drug combinations, medical science, etc., can solve the problems of high stenosis inhibition rate, low toxicity, and unproven stenosis inhibition effect, and achieve high inhibition effect and low toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] (1) Preparation of coating solution 1
[0094] Measure L-serine ethyl ester hydrochloride (CAS No. 26348-61-8) (56 mg) and paclitaxel (CAS No. 33069-62-4) (134.4 mg). Absolute ethanol (1.2 mL), tetrahydrofuran (1.6 mL), and RO (Reverse Osmosis, reverse osmosis membrane) treated water (hereinafter referred to as RO water) (0.4 mL) were added to the above components and dissolved to prepare coating solution 1 .
[0095] (2) Drug application to the balloon
[0096] A balloon catheter (manufactured by Terumo Co., Ltd., the material of the balloon (expansion portion) is nylon elastomer) with a diameter of 3.0×length 20 mm (expansion portion) was prepared. The amount of paclitaxel in coating solution 1 is about 3 μg / mm 2 coated on the inflated balloon in such a way that the solvent in the coating solution evaporates slowly. Specifically, the drug is discharged from the opening of the front end of the dispensing tube while the side surface of the front end of the dispensin...
Embodiment 2
[0099] (1) Preparation of coating solution 2
[0100] Measure L-serine ethyl ester hydrochloride (70mg) and paclitaxel (180mg). Anhydrous ethanol (1.5 mL), acetone (2.0 mL), tetrahydrofuran (0.5 mL), and RO water (1 mL) were added and dissolved to the above-mentioned components, respectively, to prepare a coating solution 2 .
[0101] (2) Drug application to the balloon
[0102] A balloon catheter (manufactured by Terumo Co., Ltd., the material of the balloon (expansion portion) is nylon elastomer) with a diameter of 3.0×length 20 mm (expansion portion) was prepared. The amount of paclitaxel in coating solution 2 is about 3 μg / mm 2 coated on the inflated balloon in such a way that the solvent in the coating solution evaporates slowly. Specifically, coating was performed in the same manner as described in Example 1 except that the drug was discharged at 0.088 μL / sec.
[0103] Thereafter, the coated balloon is dried to prepare a drug-eluting balloon.
Embodiment 3
[0105] (1) Preparation of coating solution 3
[0106] Measure L-serine ethyl ester hydrochloride (70mg) and paclitaxel (168mg). Anhydrous ethanol (1.5 mL), tetrahydrofuran (1.5 mL), and RO water (1 mL) were added and dissolved to the above-mentioned components, respectively, to prepare a coating solution 3 .
[0107] (2) Drug application to the balloon
[0108] A balloon catheter (manufactured by Terumo Co., Ltd., the material of the balloon (expansion portion) is nylon elastomer) with a diameter of 3.0×length 20 mm (expansion portion) was prepared. The amount of paclitaxel in coating solution 3 is about 3 μg / mm 2 coated on the inflated balloon in such a way that the solvent in the coating solution evaporates slowly. Specifically, coating was carried out in the same manner as described in Example 1 except that the drug was discharged at 0.101 μL / sec.
[0109] Thereafter, the coated balloon is dried to prepare a drug-eluting balloon.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com