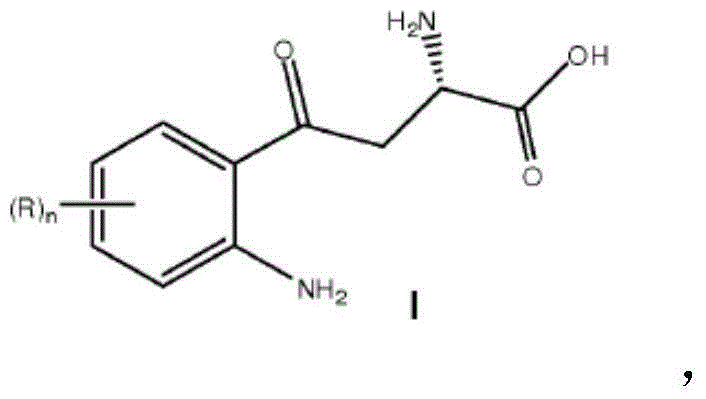
Synthesis of chiral kynurenine compounds and intermediates
A compound, chiral phosphine ligand technology, applied in the field of synthetic compounds, can solve problems such as low solubility and no success
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0160] Preparation of Z-α-acetamido-6-chloroindole-3-propenoic acid ethyl ester
[0161] Add 6-chloroindole-3-carbaldehyde (530 g, 2.95 mol), ethyl acetamidomalonate (837 g, 4.42 mol, 1.50 eq) and pyridine (2650 mL) into a 5 L 3-neck round bottom flask and stir and heated to 25°C until a clear solution formed. The resulting solution was cooled to -5°C and acetic anhydride (964 g, 9.44 mol, 3.20 equiv) was added slowly over 3-4 hours at 12. TLC monitoring (100% ethyl acetate) showed complete conversion of the N-acylated product to the desired product after stirring for 4 hours. Concentrated HCl (130 mL) was added to the slurry to adjust the pH to 5-6. The product was then filtered, washed with water and then 3 times with methyl tert-butyl ether (MTBE). 10.6 g of product were dried in a vacuum oven at 45°C and <10 mm Hg. Yield of ethyl Z-[alpha]-acetamido-6-chloroindole-3-propenoate: 407 g (1.33 mol, 45% yield, 74% corrected for recovered starting material). HPLC: 97.4% che...
example 2
[0164] Preparation of (S)-ethyl 2-acetamido-3-(6-chloro-1H-indol-3-yl)propionate
[0165] Ethyl Z-α-acetamido-6-chloroindole-3-propenoate (434 g, 1.41 mol) was dissolved in methanol at 60 °C, analytically pure (AR = anhydrous reagent grade, 6510 mL) to form a light yellow solution. The solution was then cooled to room temperature and charged to a 20 L hydrogenator. The hydrogenator was purged three times with 25 psi nitrogen and allowed to stir for 20 minutes under 25 psi nitrogen to remove any dissolved oxygen from the reaction mixture. The hydrogenator is then discharged and the chiral catalyst [(S,S',R,R')-DuanPhosRh(COD)][BF 4 ] (0.93 g, 0.0014 mol, 0.001 equiv) was added to the reaction mixture. The hydrogenator was pressurized with 30 psi of hydrogen and stirred for 10 minutes. The gas is then vented and the procedure repeated two more times. The hydrogenator was re-pressurized with 90 psi hydrogen and stirred overnight at room temperature. Periodically repressuriz...
example 3
[0168] Preparation of (2S)-2-(acetamido)-4-(2-carbonylamino-4-chlorophenyl)-4-oxobutanoic acid ethyl ester
[0169] Into a 50 gallon reactor was charged (S)-ethyl 2-acetamido-3-(6-chloro-1H-indole-3- base) propionate (3000 g, 9.716 mol), heated to 30°C to give a clear solution, then cooled to -20°C and stirred at maximum stirring speed. The pH of the solution was 10.0. A solution of MCPBA (4785 g, 27.73 mol, 2.854 equiv) in THF (2.5 L) and DCM (15 L) was prepared and added to a 20 L addition funnel. At the same time, a solution of sodium carbonate (2265 g) in water (12 L) was added to a second 20 L addition funnel. The MCPBA solution was added at a rate to maintain the temperature <-15°C. Once the reaction mixture reached a pH of 3-4, sodium carbonate solution was added to maintain the pH of the reaction mixture at 3-6. Once both solutions were added to the reactor, the progress of the reaction was checked by spotting the organic layer on a TLC plate. In 100% ethyl acetat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com