Ursolic acid-aspirin conjugate and application thereof in preparing drugs for preventing tumor metastasis
A technology for aspirin and tumor metastasis, applied in ursolic acid-aspirin conjugates and its application fields, can solve the problems of lack of selectivity, toxic and side effects of chemotherapy drugs, and reduce the efficacy of drugs, and achieve the effect of preventing and treating lung metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] At room temperature, 0.5g of acetylsalicylic acid (aspirin) was dissolved in 20mL of dry dichloromethane, 2mL of oxalyl chloride was added, magnetically stirred for 10h, and the gas and solvent were evaporated under reduced pressure. Add 20mL of dichloromethane to the reaction bottle again, add dropwise to 20mL1v / 1v pyridine:dichloromethane mixed solution dissolved with 1.27g ursolic acid and 0.1eqDMAP through the constant pressure funnel, and continue stirring for 8-10h after the dropwise addition is completed . After the reaction was complete, dichloromethane was distilled off under reduced pressure. Add 100mL water to the reaction bottle to precipitate the product, filter it with suction, wash the filter cake with 500mL water until neutral, dry it in vacuum, and get ASP-UA by column chromatography.
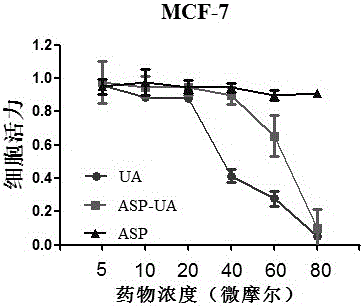
[0030] Properties: white powder; yield: 70.51%; product characterization data are as follows: 1HNMR (500MHz, CDCl3) δ10.52 (s, 1H), 7.93 (dd, J=8.0, 1.6Hz, 1H), 7.55–7....
Embodiment 2
[0034] After digesting mouse breast cancer cells MCF-7 in the logarithmic growth phase, the cell density was adjusted to 1×10 5 cells / mL, seeded in a 96-well plate, 100 μl per well, placed at 37°C, 5% CO 2 Cultivate in the incubator for 24 hours; remove the old medium, add the medium for the test drug to dilute the stock solution of the test drug, set different concentrations, 100 μl per well, and set up a blank control group, and set 5 replicates in each group. hole. After 24 hours of drug action, discard the drug-containing medium, add 100 μl of blood-free phenol red-free 1640 medium to each well, then add 100 μl of 0.5 mg / ml MTT solution, and continue incubation for 4 hours, then terminate the culture; carefully aspirate and discard 96 Add 100 μl DSMO to each well of the supernatant of the well plate, shake for 10 minutes, measure the light absorption value (OD value) of each well on a microplate reader at a wavelength of 570 nm, and calculate the cell survival rate (%) = ...
Embodiment 3
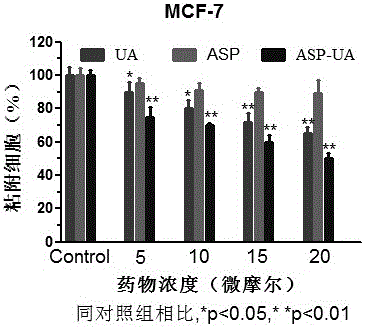
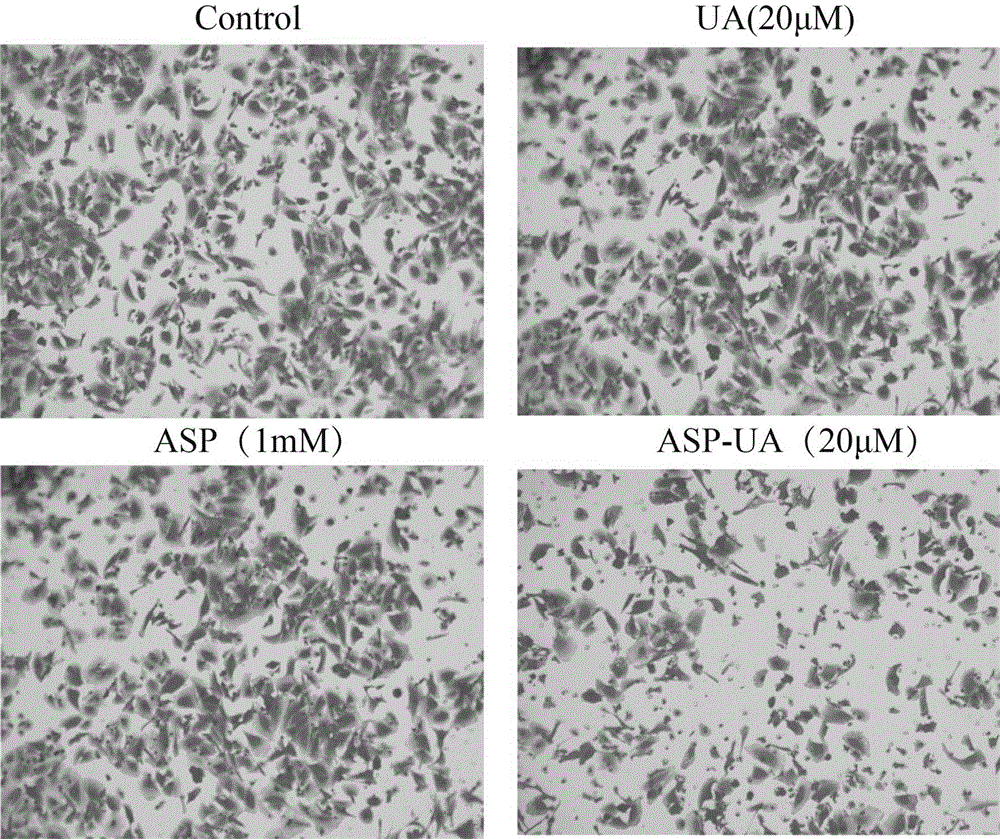
[0037] The fibronectin FN (fibronectin) stored at -20°C was placed in a 37°C water bath until it was completely melted, and the serum-free culture medium was used to prepare the FN working solution with a concentration of 10 μg / ml. Use 100 μL / well FN working solution to completely cover the 96-well plate, leave it at room temperature overnight, and gently suck off the liquid. Take MCF-7 cells in the logarithmic growth phase and make a single cell suspension with a cell concentration of 5×10 5 / ml, mixed with different concentrations of drugs (0, 5, 10, 15, 20 μ M), inoculated in FN-coated 96-well plates. 37℃,5%CO 2 After incubation for 2 hours, wash lightly with PBS 3 times, add 100 μl of MTT culture solution after drying, continue to incubate for 4 hours, discard the supernatant, add 100 μl DMSO, and detect the OD value at 570 nm with an enzyme-linked detector after dissolution. Adhesion inhibition rate (%)=(1-average OD value of medication group / average OD value of blank c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com