Application of 23-trans-para-coumaroyl tormentic acid compound
A technology of coumaryl compounds, applied in the field of botanical fungicides, can solve the problem of no biological activity research, etc., and achieve the effects of good inhibitory activity, low pesticide residues, and good environmental compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
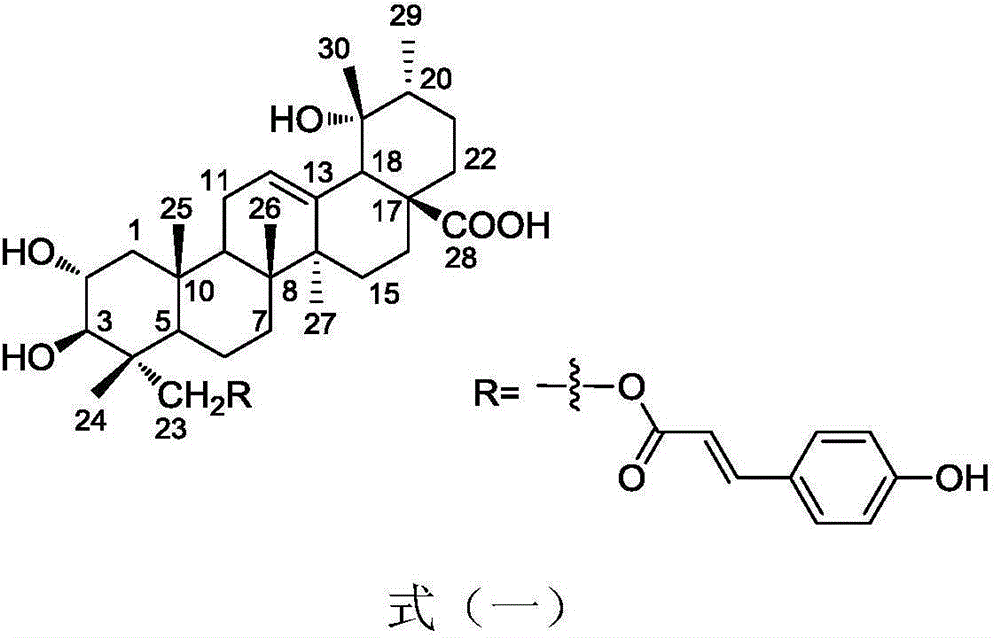
[0012] 23-trans-p-coumaroyltormentic acid represented by formula (1) is a known compound of ursane type.
[0013] The preparation process of 23-trans-p-coumaroyl polinic acid shown in formula (1) can be referred to in literature deTommasiN, deSimoneF, PizzaC, etal. 8): The description in 1067-1073 is carried out.
Embodiment 2
[0014] Embodiment 2: antibacterial activity test
[0015] The microdilution method was used to determine the antibacterial activity of the compound shown in formula (1) against Botrytis cinerea ("From Natural Products to New Pesticide Development-Principle and Method", Wu Wenjun, 2006, Chemical Industry Press).
[0016] 1) Preparation of bacterial suspension
[0017] Inoculate the fungus to be tested on the surface of PDA medium and culture at 28°C for 72 hours, draw 2 mL of sterile 0.85% NaCl solution (containing 0.25% Tween-20) to wash the culture, and gently scrape off the colony with a glass scraper. Draw an appropriate amount of bacterial suspension into a sterile test tube and adjust to 0.5 McFarland turbidity (equivalent to 1.5×10 8 CFU / mL), spare;
[0018] 2) Sample preparation
[0019] Take 1 mg of the sample to be tested (compound of formula (1)) and positive control (amphotericin B) respectively, dissolve in 100 μ D DMSO, mix well, draw 50 μ L of the sample solut...
Embodiment 3
[0026] Embodiment 3: wettable powder
[0027] Formula (weight ratio): 20% of 23-trans-p-coumaroyl-poisonic acid powder, 20% of white carbon black, 5% of sodium lauryl sulfate, 1.0% of CMC and 3.0% of pull-off powder, Attapulgite residue.
[0028] Preparation method: Mix the above-mentioned raw materials evenly, control the processing temperature at 0-50°C, and pulverize them to more than 150 mesh in a jet mill or other high-mesh mills, control the water content at 6-8% (mass percentage), pH Control it at 7-7.5.
[0029] How to use: Spray with 600 times liquid, and spray evenly on the leaves of the plant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com