Application of Astemizole and salts or solvates thereof in preparing drug for preventing and/or treating malignant lymphoma
A technology for malignant lymphoma and astemizole, applied in the field of medicine, can solve the problems of poor tolerance of high-intensity chemotherapy drugs and an average survival rate of less than 50%, and achieve the effect of preventing and treating malignant lymphoma diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
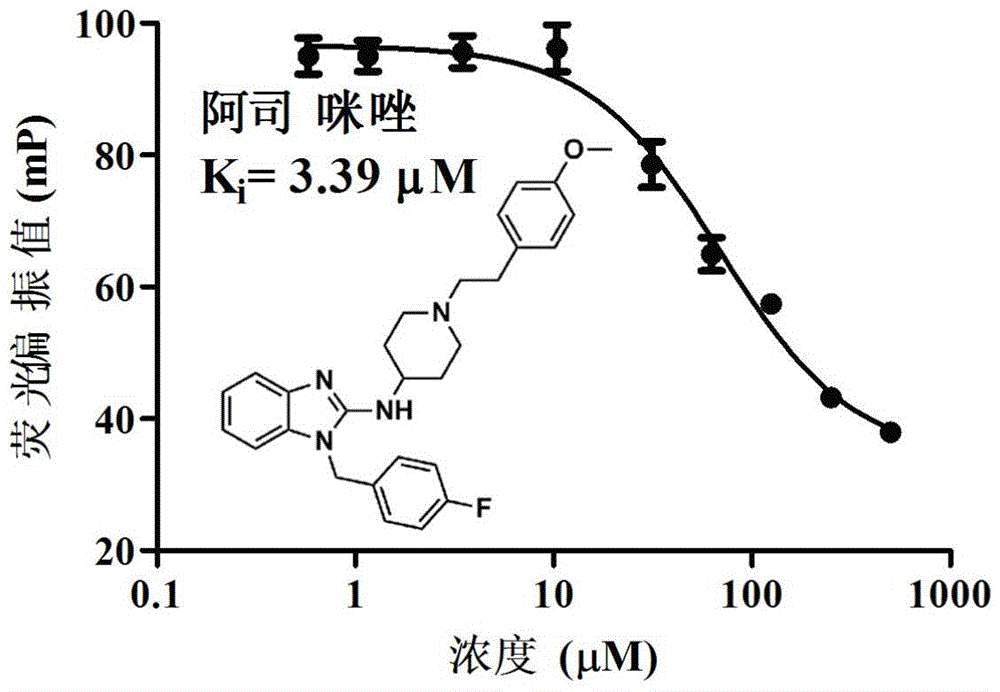
[0046] Example 1: Fluorescence Polarization (FP) detection of the effect of astemizole on EZH2-EED interaction
[0047] For fluorescence polarization experiments, EHZ2 polypeptide labeled with fluorescein isothiocyanate (FITC) was used as a tracer substrate. Add 20 μM MEED protein, 5 μM tracer substrate and different concentrations of astemizole compound to 100 μl buffer (25mMBISTRIS, 500mMNaCl, 1mMDTT), incubate at room temperature for 5 hours, and use a fully automatic multifunctional microplate reader (POLARstar OmegaMicroplatereader, purchased from BMG .LABTECH) to detect the fluorescence polarization value. The excitation and emission wavelengths used were 480 nm and 520 nm, respectively. Three groups of repeated experiments were set up in each group, and the experimental data were analyzed and drawn using GraphPad Prism 5.0 software. The experimental results are as follows figure 1 shown. This result indicates that astemizole can compete with EZH2 polypeptide for bind...
Embodiment 2
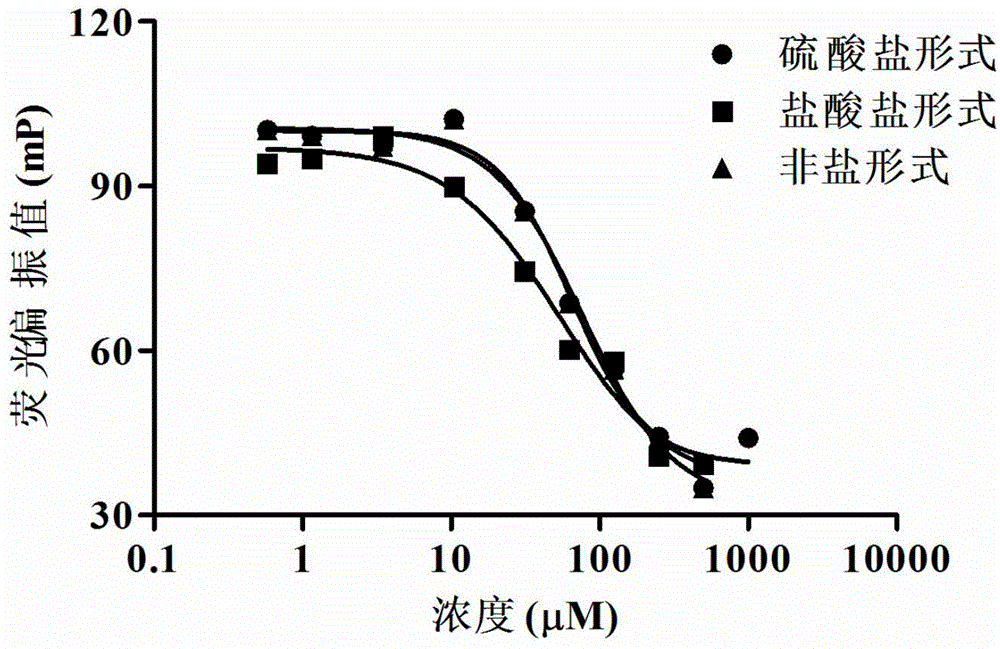
[0048] Embodiment 2: Fluorescence polarization method (FP) detects the influence of astemizole salt on EZH2-EED interaction
[0049] FITC-labeled EHZ2 polypeptide was used as tracer substrate. In 100μl buffer (25mMBISTRIS, 500mMNaCl, 1mMDTT), add 20μM EED protein and 5μM tracer substrate and different concentrations and different forms of astemizole salt or non-salt form compounds, namely sulfate, hydrochloride and For compounds in non-salt form, after incubation at room temperature for 5 hours, the fluorescence polarization value was detected with a fully automatic multi-functional microplate reader. The excitation and emission wavelengths used were 480 nm and 520 nm, respectively. Three groups of repetitions were set up for each group of experiments, and the experimental data were analyzed and graphed using GraphPad Prism 5.0 software. The experimental results are as follows figure 2 shown. The results indicated that the salt form of astemizole could also compete with EZ...
Embodiment 3
[0050] Example 3: Using the protein thermal stability shift method (ThermalShift) to detect the effect of astemizole on the thermal stability of EED protein.
[0051] Protein thermal stability migration experiment, using RT-PCR instrument (7500FastRT-PCRSystem (ABI)) to measure protein thermal stability, using Bis-ANS as a fluorescent dye to monitor the change of the fluorescence value of the system, and setting the excitation light and emission wavelength respectively for 395nm and 492nm. Add 20 μM EED protein, 50XBis-ANS dye and astemizole compound in different concentrations in 20 μl buffer system (25mMBISTRIS, 500mMNaCl, 1mMDTT). At a heating rate of 1%, the temperature of the system was gradually raised from 25°C to 95°C, and the change of fluorescence intensity was recorded at the same time, and the dissolution temperature (Tm) of EED protein at different compound concentrations was calculated by Boltzmann fitting method. All experiments are all set as three repetition ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com