Pharmaceutical composition for inhibiting immune response through inducing differentiation into regulator t cells and promoting proliferation of regulator t cells
A technology for suppressing immune response and composition, which is applied in the direction of drug combination, cell culture active agent, active ingredient of heterocyclic compound, etc., and can solve problems such as the effectiveness of suppressing immune response that has not been mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
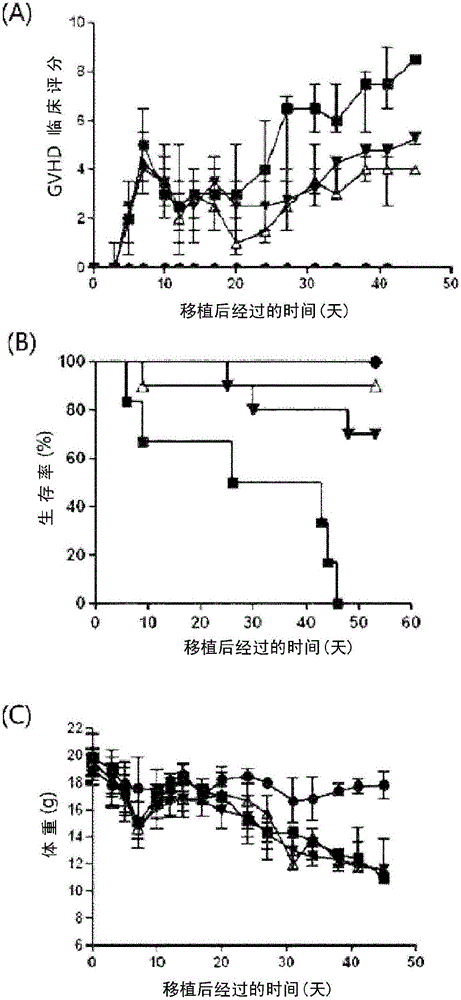
[0065] Example 1: Establishment of Allogeneic Immune Response Mouse Model
[0066] 6-8 week-old C57BL / 6 (H-2kb) mice and BALB / c (H-2kd) mice having different MHC classes from each other were purchased from OrientBioCo., Ltd. . The mice were used under their care after passing the regulations of the Administrative Committee of the Experimental Animal Department of The Catholic University of Korea. Recipient mice BALB / c (H-2kd) were irradiated with 800 cGy of whole body radiation. Within 24 hours, 5 × 10 cells isolated from donor mice C57BL / 6 (H-2kd) 6 bone marrow cells and 5 x 10 6 Spleen cells were transplanted intravenously into allogeneic recipient mice to induce a graft-versus-host rejection model. The bone marrow-transplanted mice were observed and evaluated for body weight, posture, activity, hair condition, and skin density (on a total 10-point scale with 2-point evaluation criteria for each item) (Table 1).
[0067] [Table 1]
[0068] score
Embodiment 2
[0069] Embodiment 2: flow cytometry (FACS analysis)
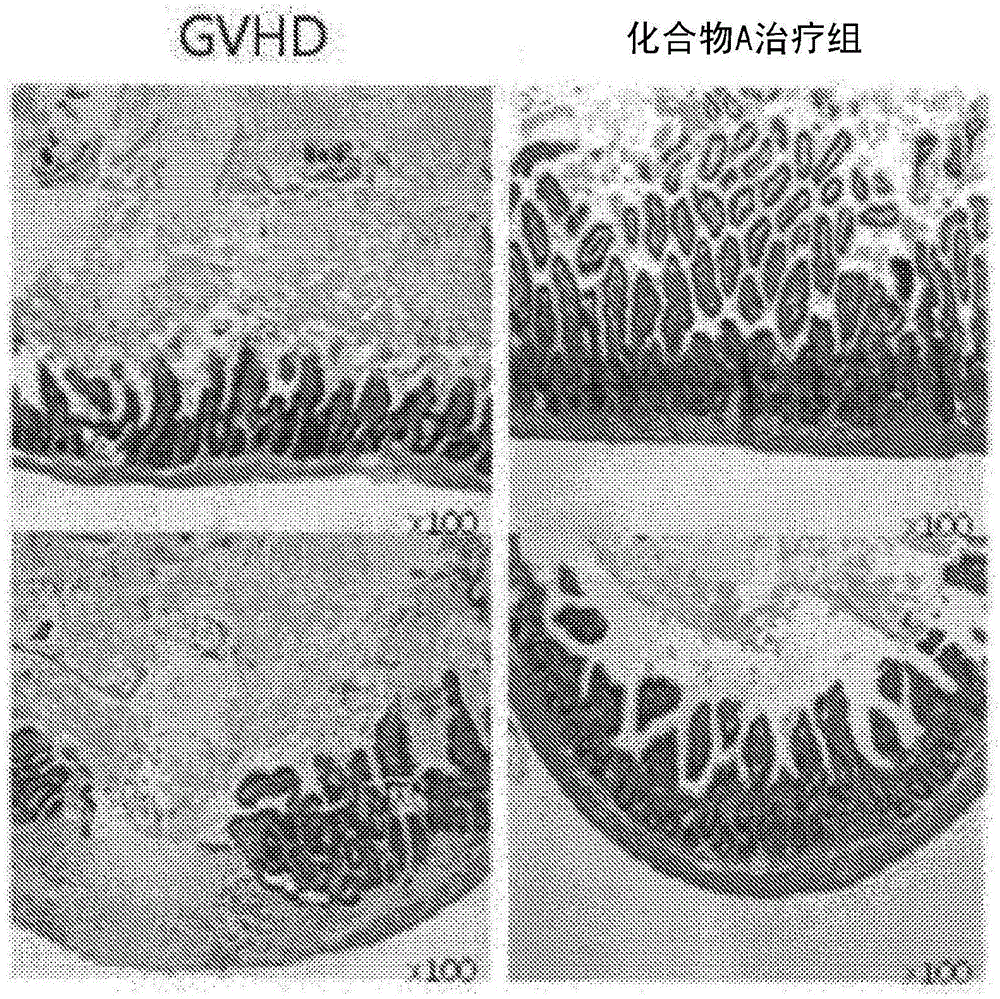
[0070] Mice were divided into groups in which (tetrahydropyran-4-yl)-[2-phenyl-5-(1,1-dioxo-thiomorpholin-4-yl)methyl-1H-indole- 7-yl]amine (LGLifeSciences Ltd., Daejeon, Korea, hereinafter referred to as "Compound A") treated GVHD group was used as a test group, and the GVHD group not treated with Compound A was used as a control group. Spleens from each group were isolated, followed by single cell isolation. The expression of CD4+CD25+Foxp3+, which is the phenotype of regulatory T cells, and CD4+IFN-γ cells and CD4+IL-4 cells were analyzed by using fluorescent monoclonal antibodies and flow cytometry.
Embodiment 3
[0071] Embodiment 3: the measurement of reactive oxygen species (ROS)
[0072] Animals in each group were sacrificed and their spleens were isolated. After isolation of single cells, the obtained cells were washed twice with phosphate-buffered saline (PBS). The reaction with 3 μM carboxy-H2DCFDA reagent was carried out at 37° C. for 10 minutes. After washing with PBS, analysis was performed with a flow cytometer at 540 nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com