Isonucleoside or isonucleoside and 2'-deoxyinosine modified tenascin-C aptamer GBI-10 as well as preparation method and application thereof
A nucleic acid aptamer and tenascin technology, applied in the field of biomedicine, can solve the problems of shortened action distance, increased action distance, and reduced activity, and achieve the effects of improved inhibition, high synthesis efficiency, and good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Solid phase synthesis of GBI-10 incorporated by isonucleoside or isonucleoside combined with 2'-deoxyinosine
[0062] DNA was synthesized using Appllied Biosystems model 394 DNA solid-phase synthesizer.
[0063]Normal deoxynucleoside phosphorylated monomers (dT, dGAc, dABz, dCAc) were purchased from Shanghai Gemma Pharmaceutical Technology Co., Ltd.; CPG (CPG-dG), CAP-A and CAP-B, oxidation I 2 Liquid, Cl 3 CCOOH was purchased from Beijing Aoke Biotechnology Company; 0.25M 5-Ethylmercapto 1H-tetrazolium solution was purchased from Shanghai Zhiyan Technology Co., Ltd. (Shanghai).
[0064] According to the method of literature (HWYu, LRZhang, JCZhuo, LTMa, LHZhang, Bioorg.Med.Chem., 1996,40,609-614), the isonucleoside compounds shown in Chemical Formula I / Chemical Formula II are prepared into Chemical Formula IV / Chemical Formula V respectively The isonucleoside phosphoramidite monomer shown. That is: vacuum-dry the monomer compound I / II, add 3.5 times the equ...
Embodiment 2
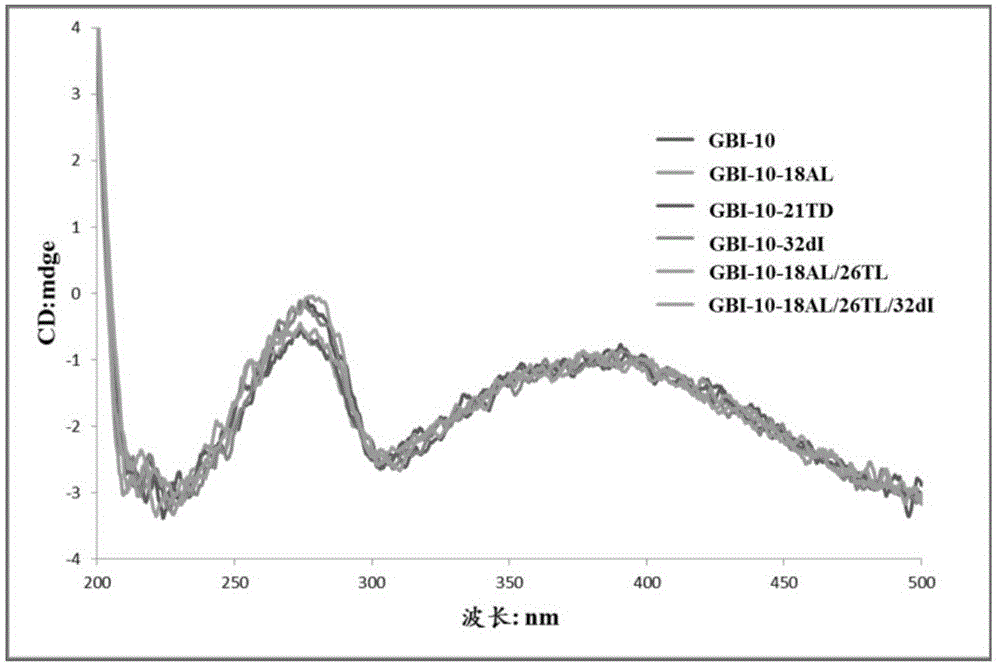
[0081] Research on the Basic Properties of the Modified GBI-10 Sequence in Example 2
[0082] 1. Sample name: The 18th, 21st, 26th or 32nd position of the GBI-10 sequence is mixed with the isonucleoside shown in the chemical formula I or the chemical formula II or the 2'-deoxyinosine shown in the chemical formula III (wherein Base is selected from The modified GBI-10 sequence obtained by coupling thymine T) instead of the natural nucleoside at the corresponding position was prepared according to the method in Example 1.
[0083] GBI-10-18A L : Incorporate the isonucleoside shown in the chemical formula I at the 18th position of the sense chain of GBI-10 to replace the natural adenine nucleoside;
[0084] GBI-10-21T D : The 21st position of the sense chain of GBI-10 incorporates the isonucleoside shown in chemical formula II instead of thymidine;
[0085] GBI-10-32dI: 2'-deoxyinosine represented by chemical formula III is incorporated into the 32nd position of the sense stra...
Embodiment 3E
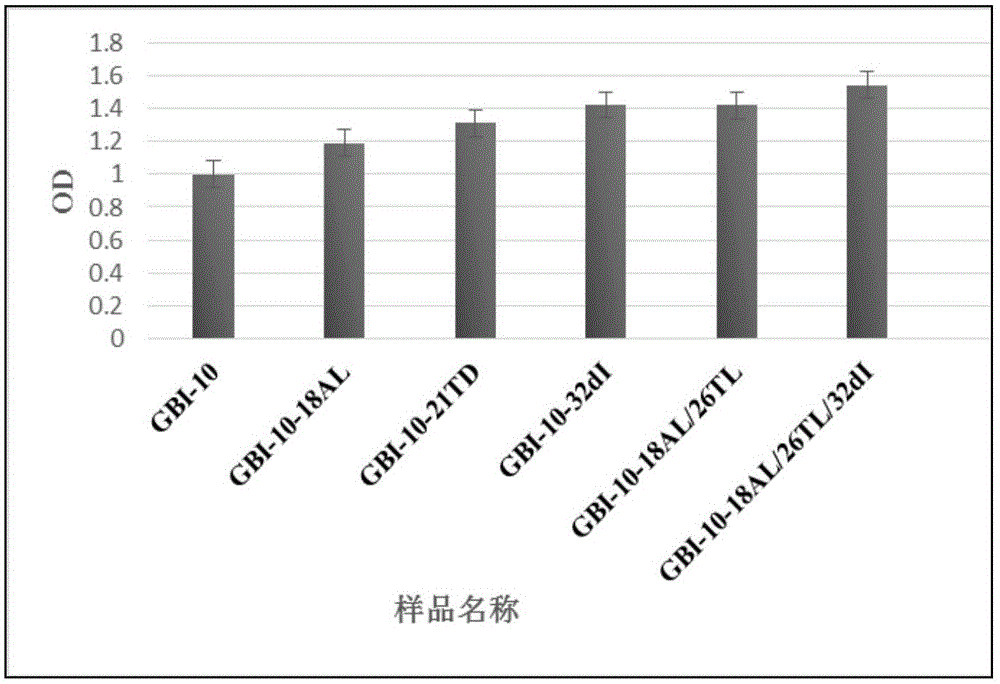
[0091] Example 3 ELISA Determination of the Affinity of GBI-10 Sequence Modified by Ionucleosides and Modified Ionucleosides Combined with Deoxyinosine and Tenascin C
[0092] 1. Sample name: GBI-10-18A L 、GBI-10-21T D , GBI-10-32dI, GBI-10-18A L / 26T L and GBI-10-18A L / 26T L / 32dI, prepared according to the method of Example 1.
[0093] 2. Method
[0094] (1) Dissolve 50 pmol TN-C protein to 100 μL, dissolve it in a solution with pH 9.6, add it to a 96-well plate, and incubate overnight at 4°C to fully bind the protein to the plate. Note: Dilute the sample in the sample tank, and inject the gun into each well to ensure that the three replicate wells of the same sample are parallel.
[0095] (2) Aspirate the protein solution, wash once with blocking solution, then fill each well with blocking solution, and block for one hour. Blocking solution: 1% BSA in PBST.
[0096] (3) Sample preparation: ssDNAAptamer dry powder (1nM) centrifuged at 36,000 rpm for 10min, diluted ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com