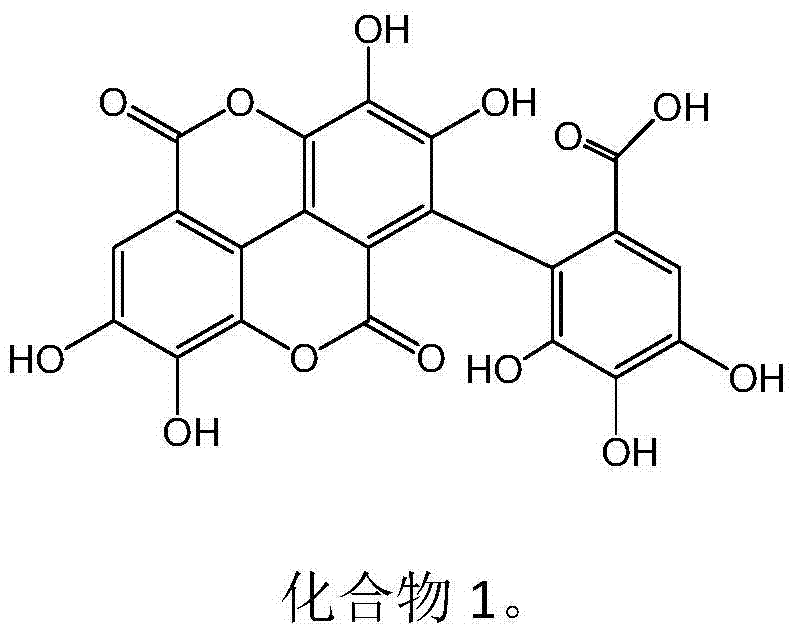
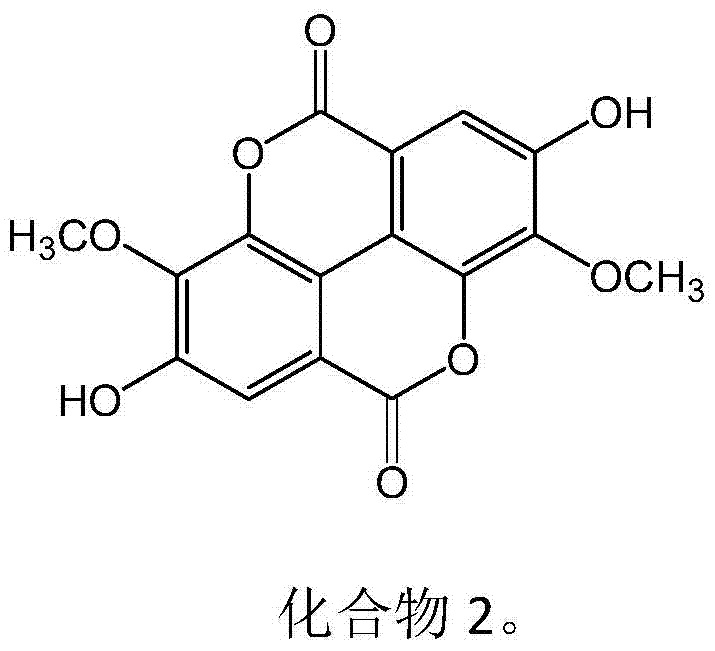
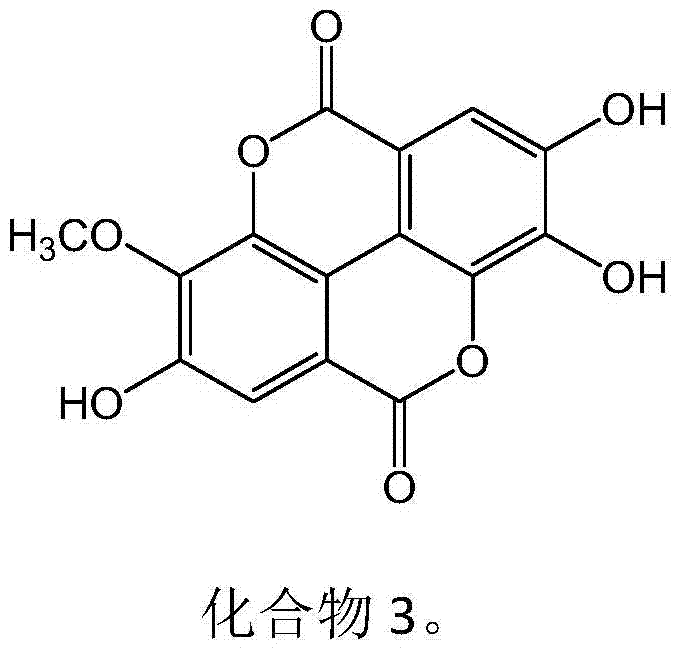
New use of ellagic acid compounds in the preparation of drugs for the treatment of hyperuricemia
A technology for hyperuricemia and compounds, applied in the field of chemical medicine, can solve the problem of few types of ellagic acid derivatives, and achieve the effects of no toxic and side effects, lowering serum uric acid level and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 The extraction and characterization of compound 2-5 of the present invention
[0037] Take 5kg of the Herba Myrobalan medicinal material, crush it, and reflux extract it with 50L of 95% ethanol for 3 times, each time for 3 hours, combine the extracts, concentrate under reduced pressure at 40°C, extract the extract with n-hexane, remove the lipids, and use Sephadex LH-20 was used for column chromatography, and 0%, 5%, 10%, 20%, 30%, 50%, and 100% ethanol were used for gradient elution and each eluted fraction was collected, and 5%, 10% , 20%, 30%, and 50% of the eluted components were concentrated under reduced pressure to remove the solvent, and then used reversed-phase C18 chromatographic columns for column chromatography, using 0%, 5%, 25%, 50%, 100% containing 2% Elute with methanol in acetic acid, collect the eluate, concentrate under reduced pressure to remove the solvent, and obtain four compounds 2-5, which are detected by HPLC-ESI-MS and NMR. The detec...
Embodiment 2
[0050] Example 2 Extraction and characterization of compounds 5 and 6 of the present invention
[0051] Take 2kg of Myrobalan medicinal material, grind it, and extract it with 20L of 95% ethanol for 3 times, each time for 3 hours, combine the extracts, concentrate under reduced pressure at 40°C, extract the extract with n-hexane, remove lipids, and use Sephadex LH ‐20 for column chromatography, respectively with 5%, 15%, 25%, 35%, 60%, 100% ethanol for gradient elution and collect each eluted fraction, 5%, 15% eluted fraction After concentrating under reduced pressure to remove the solvent, use reversed-phase C18 column chromatography to carry out purification respectively, use 5%, 15%, 25%, 50, 100% methanol containing 2% acetic acid to carry out elution, collect eluate, reduce Concentrated under pressure to remove the solvent, two compounds 5 and 6 can be obtained, detected by HPLC-ESI-MS and NMR, the detection and characterization data of compounds 5 and 6 are as follows: ...
Embodiment 3
[0059] Example 3 Extraction and Characterization of Compounds 7,8,9 of the present invention
[0060] Take 3 kg of Chinese tallow tree medicinal material, crush it, and reflux extract it with 30 L of 95% ethanol for 3 times, each time for 3 hours, combine the extracts, concentrate under reduced pressure at 40 ° C, and use petroleum ether, ethyl acetate and n-butanol for the extract respectively. After extraction, the ethyl acetate extraction part was concentrated under reduced pressure to remove the solvent, and then separated by silica gel column chromatography, using chloroform-methanol gradient elution (V 氯仿 :V 甲醇 =98:2—70:30), the obtained part was recrystallized and purified to obtain compounds 7, 8, and 9. Detected by HPLC-ESI-MS and NMR, the detection and characterization data of compounds 7, 8, and 9 are as follows:
[0061] Compound 7: 3,3’,4’‐trimethoxyellagic acid C 17 h 12 o 8 [M‐H ‐ ]:343.2
[0062] 1 H‐NMR (DMSO‐d 6 ,300MHz)δ:7.55(1H),7.64(1H),4.05(3H),4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com