Preparation method and application of pentacyclic triterpene derivatives
A compound and derivative technology, which is applied in the preparation and application field of pentacyclic triterpenoid structure derivatives, can solve the problems of poor targeting and high production cost of psoriasis drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of gels with compounds of formula (II-VII)
[0028] Get a certain amount of any one of the compounds with molecular formula (II-VII) to make a gel according to the following formula:
[0029] Any one of the compounds of formula (II-VII) 100 mg
[0030] Carbomer 400 mg
[0031] Glycerin 550mg
[0032] Propylene glycol 650mg
[0033] Parabens 100 mg
[0034] Triethanolamine 200 mg
[0035] Add water to 5ml.
[0036] Preparation:
[0037] Weigh any one of the compounds with molecular formula (II-VII) in the prescribed amount into the beaker, add the prescribed amount of carbomer into it, let it fully swell overnight, and then add glycerin, propylene glycol, antiseptic agent, water and triethanolamine, and stir evenly to make the pH value of the composition 5.8.
Embodiment 2
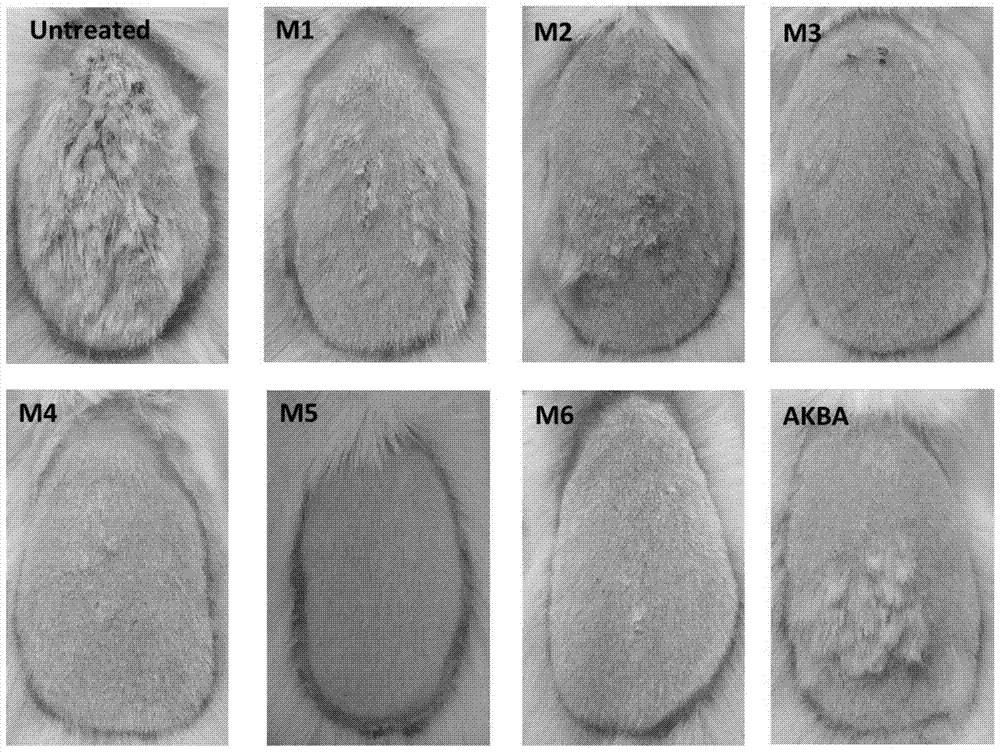
[0038] Embodiment 2: The compound of molecular formula (II-VII) inhibits the test of NF-kappa B pathway in vivo ( figure 1 )
[0039] Luciferase (luciferase) transgenic mice of NF-kappa B, 6 mice in each group, totally 8 groups Control (Ctr, blank gel group), M1 treatment group, M2 treatment group, M3 treatment group, M4 treatment group, M5 treatment group group, M6 treatment group and AKBA treatment group. Mice were first induced with imiquimod for 7 days. After treatment of "psoriatic" mice with 0.5% M1-M6 and 0.5% AKBA gel 4 times (once a day) starting from day 8, they were imaged with a small animal intravital imaging system. Through analysis, it is found that 0.5% of the compound with molecular formula (II-VII) inhibits the activation of NF-kappa B more effectively than 0.5% of AKBA.
Embodiment 3
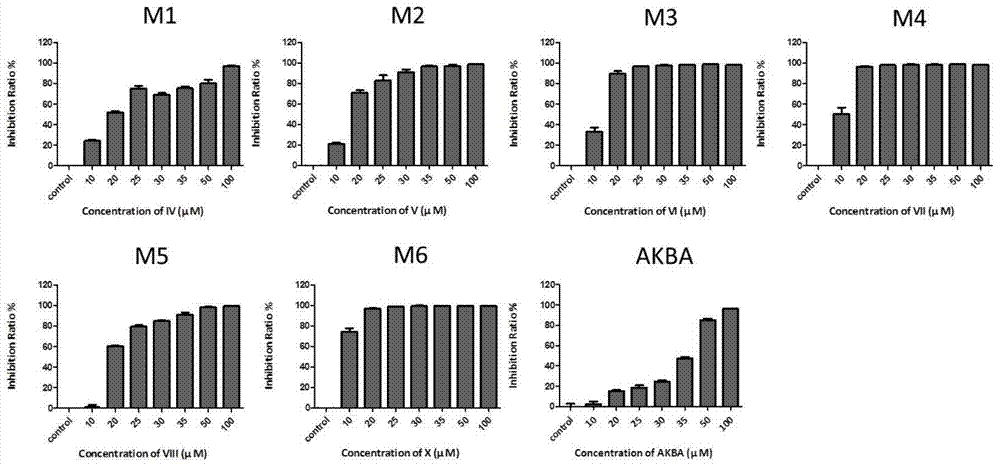
[0040] Embodiment 3: The test that the compound with molecular formula (II-VII) inhibits the growth of keratinocytes in vitro ( figure 2 )
[0041] Take the primary mouse cells in the logarithmic growth phase in a 96-well plate, 2×10 per well 4 AKBA derivatives and AKBA in gradient concentrations were added to each cell, with a total volume of 100 μl, DMSO with a final concentration of 0.25% was added to the control group, and only 100 μl medium was added to the blank control wells, and three replicate wells were set up in each group. After 24 hours of culture in the cell incubator, add 10 μl of CCK-8 solution to each well, and place the culture plate in the incubator (37°C, 5% CO 2 conditions) and incubate for 4 hours in the dark. The O.D. value at the wavelength of 450nm was measured by a microplate reader. Calculation of inhibition rate: inhibition rate = [1-(O.D. value of experimental group / O.D. value of control group)]×100%. Among them, the compound with the molecula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com