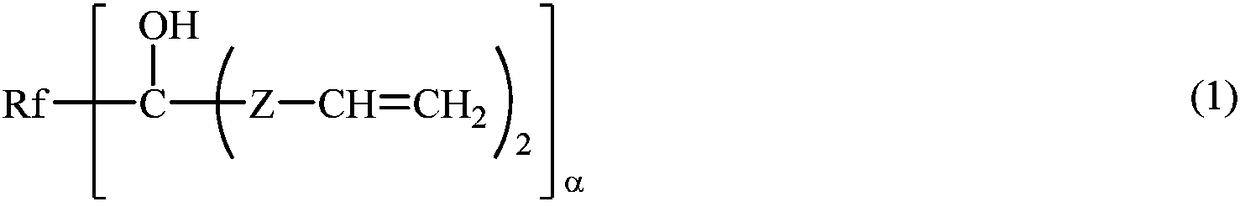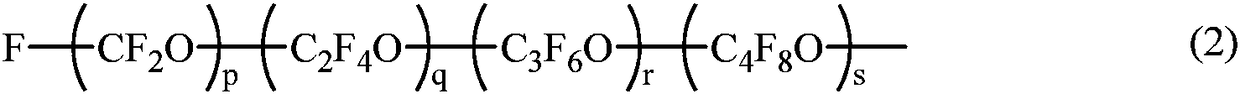Fluoropolyether-containing polymers
A technology of polymers and fluoropolyethers, which is applied in the field of polymers containing fluoropolyethers, can solve the problems of lack of weather resistance, achieve excellent water/oil repellency, and maintain antifouling effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] 150 g of tetrahydrofuran and 300 g of 1,3-bistrifluoromethylbenzene were charged into the reactor, and 160 ml of 0.7M allylmagnesium bromide was added dropwise thereto. Next, slowly add 300g (4.8×10- 2 mol) a compound of the following formula (I).
[0132]
[0133] pl:q1=47:53, pl+q1≈43
[0134] The resulting solution was heated at 60 °C for 4 hours. It was then cooled to room temperature and added dropwise to 300 g of 1.2M aqueous hydrochloric acid to terminate the reaction. The lower layer or fluorine compound layer was recovered by separation operation and washed with acetone. The washed lower layer or fluorochemical layer is recovered again. The residual solvent was distilled off in vacuo to obtain 292 g of a fluoropolyether-containing polymer of the following formula (II).
[0135]
[0136] p1:q1=47:53, p1+q1≈43
[0137] use 1 The polymer was analyzed by H-NMR, and the results are shown below.
[0138] 1 H-NMR
[0139] δ2.2(-CO H (CH 2 CH=CH 2 ) ...
Embodiment 2
[0144] 150 g of tetrahydrofuran and 300 g of 1,3-bistrifluoromethylbenzene were charged into the reactor, and 320 ml of 0.7M allylmagnesium bromide was added dropwise thereto. Next, slowly add 300g (9.6×10- 2 mol) a compound of the following formula (III).
[0145]
[0146] pl:qI=47:53, pl+q1≈43
[0147] The resulting solution was heated at 60 °C for 4 hours. It was then cooled to room temperature and added dropwise to 300 g of 1.2M aqueous hydrochloric acid to terminate the reaction. The lower layer or fluorine compound layer was recovered by separation operation and washed with acetone. The washed lower layer or fluorochemical layer is recovered again. The residual solvent was distilled off in vacuo to obtain 286 g of a fluoropolyether-containing polymer of the following formula (IV).
[0148]
[0149] pl:q1=47:53, pl+q1≈43
[0150] use 1 The polymer was analyzed by H-NMR, and the results are shown below.
[0151] 1 H-NMR
[0152] δ2.2(-CO H (CH 2 CH=CH 2 ...
Embodiment 3
[0157] 150 g of tetrahydrofuran and 300 g of 1,3-bistrifluoromethylbenzene were charged into the reactor, and 160 ml of 0.7M allylmagnesium bromide was added dropwise thereto. Next, slowly add 300g (4.8×10 -2 mol) a compound of the following formula (V).
[0158]
[0159] The resulting solution was heated at 60 °C for 4 hours. It was then cooled to room temperature and added dropwise to 300 g of 1.2M aqueous hydrochloric acid to terminate the reaction. The lower layer or fluorine compound layer was recovered by separation operation and washed with acetone. The washed lower layer or fluorochemical layer is recovered again. The residual solvent was distilled off in vacuo to obtain 277 g of a fluoropolyether-containing polymer of the following formula (VI).
[0160]
[0161] use 1 The polymer was analyzed by H-NMR, and the results are shown below.
[0162] 1 H-NMR
[0163] δ2.2(-CO H (CH 2 CH=CH 2 ) 2 )1H
[0164] δ2.3(-COH(C H 2 CH=CH 2 ) 2 )4H
[0165] δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com