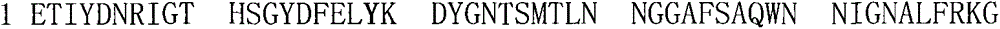Recombinant basic xylanase resistant to chelating agent ethylenediamine tetraacetic acid and construction method thereof
A xylanase and alkaline technology, which is applied in the field of industrial enzyme development and utilization, can solve the problems of enzyme activity reduction and achieve the effects of saving process water, reducing use, and huge ecological benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The invention discloses a group of EDTA-resistant alkaline xylanase and its encoding gene, which is characterized in that the alkaline xylanase gene xynA contained in GenBank accession number EU421717.1 corresponds to the amino acid sequence of xylanase xynA The accession number is ACA00160.1, except the 27 amino acid signal peptide of the N-segment, the remaining 201 amino acids are the mature fragment XynA-M of the xylanase, and the corresponding mature fragment of the xylanase gene is xynA-M . For xynA-M, design point mutation primers (FP1, RP1, FP2, RP2, FP3, RP3) for site-directed mutagenesis, and change the guanine G at positions 56 and 57 of the published gene into adenine A and thymine T, respectively , change the guanine G at positions 56 and 57 to thymine T and cytosine C respectively, and change the thymine T and guanine G at positions 55, 56 and 57 to cytosine C, adenine A and cytosine C respectively , so that tryptophan W (TGG) at the 19th position of the ...
Embodiment 2
[0071] Medium: LB resistant medium: tryptone 10g / L, yeast extract 5g / L, NaCl 10g / L, kanamycin 50μg / mL;
[0072]Culture method: the recombinant strains BL21(DE3) / pET28a-xynA-M-W19Y, BL21(DE3) / pET28a-xynA-M-W19F and BL21(DE3) / pET28a-xynA-M-W19H in Example 1 were grown in LB After activating and preparing seeds on the resistant plate, transfer them to liquid LB medium at an inoculum size of 1:100, and cultivate to OD at 37°C and 200rpm. 600 If the concentration is 0.6-0.8, add the inducer isopropylthiogalactoside (IPTG) to a final concentration of 0.5mM, and then culture at 18°C and 200rpm for 8 hours.
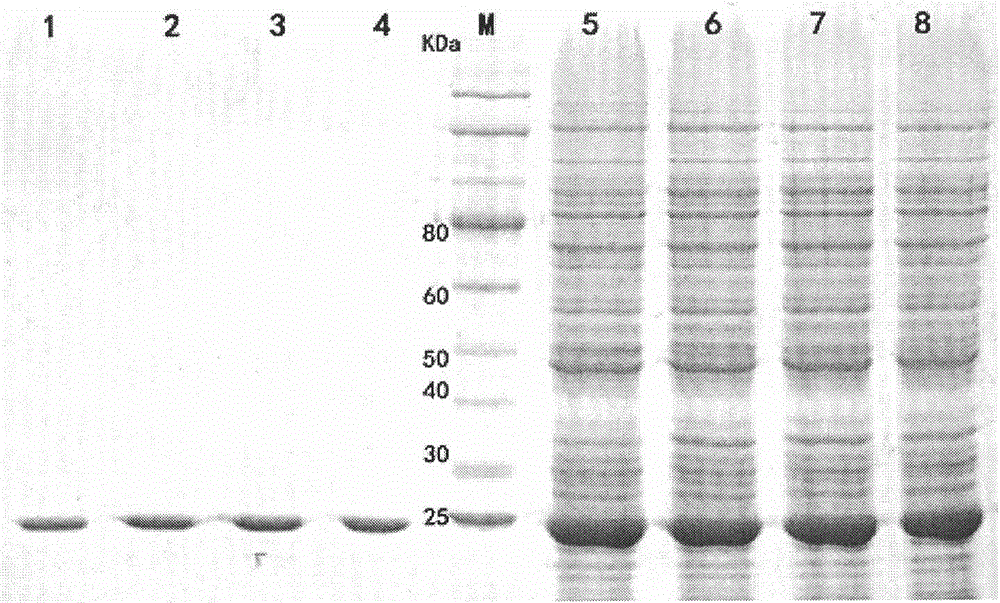
[0073] The induced bacterial cells were ultrasonically destructed, passed through a HIS column for purification and concentration, and purified xylanase mutants XynA-M-W19Y, XynA-M-W19F and XynA-M-W19H were obtained.
Embodiment 3
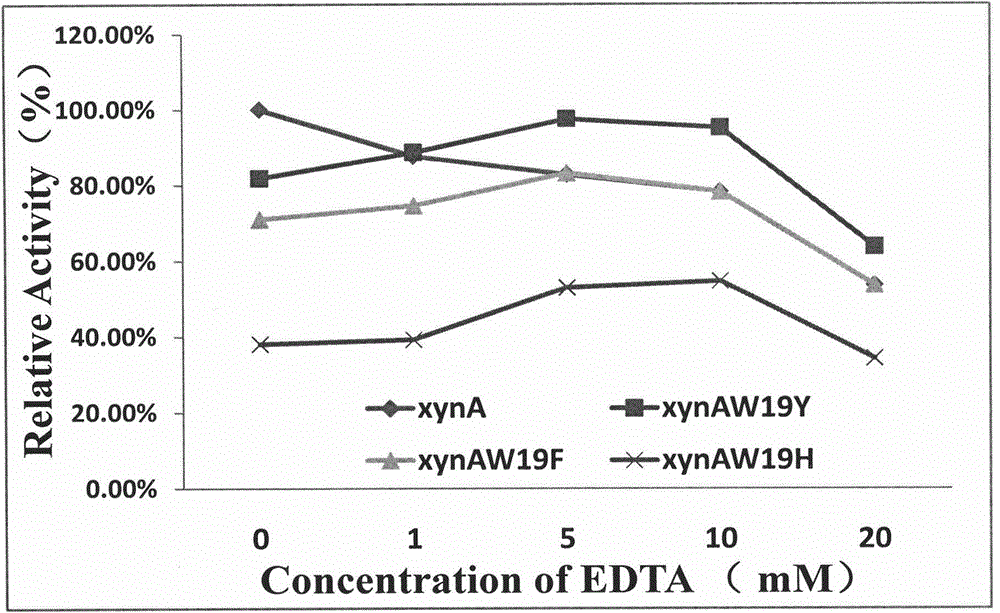
[0075] Method for measuring xylanase enzyme activity: Take 20 μl of diluted enzyme solution, add 380 μl of 1% xylan (beech xylan, pH9.2 glycine-NaOH buffer solution) solution, mix well, and place at 60°C for 10 minutes. Immediately add 600 μl 3,5-dinitrosalicylic acid (DNS), boil for 7 minutes, measure OD 540 nm value. The blank control is the first inactivated enzyme solution (that is, first take 20 μl of the enzyme solution and inactivate it in a water bath at 100°C for 5 minutes, then add the substrate to react for 10 minutes). The enzyme activity unit (U) was defined as the amount of enzyme needed to catalyze the production of 1 μmol xylose per minute.
[0076] Enzyme activity calculation formula: A=(M*V1*C) / (V2*T)
[0077] In the formula: A——enzyme activity (U / ml)
[0078] M——enzyme solution dilution multiple
[0079] V1——enzyme reaction volume (ml)
[0080] C——xylose concentration (μmol / ml), which can be calculated according to xylose standard curve and measured OD5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com