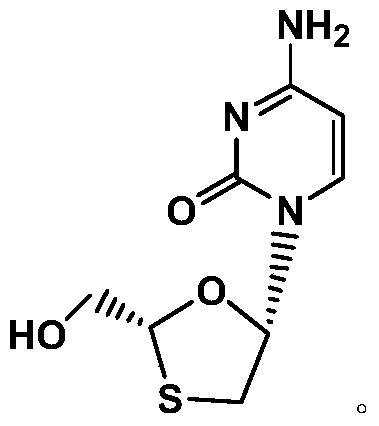
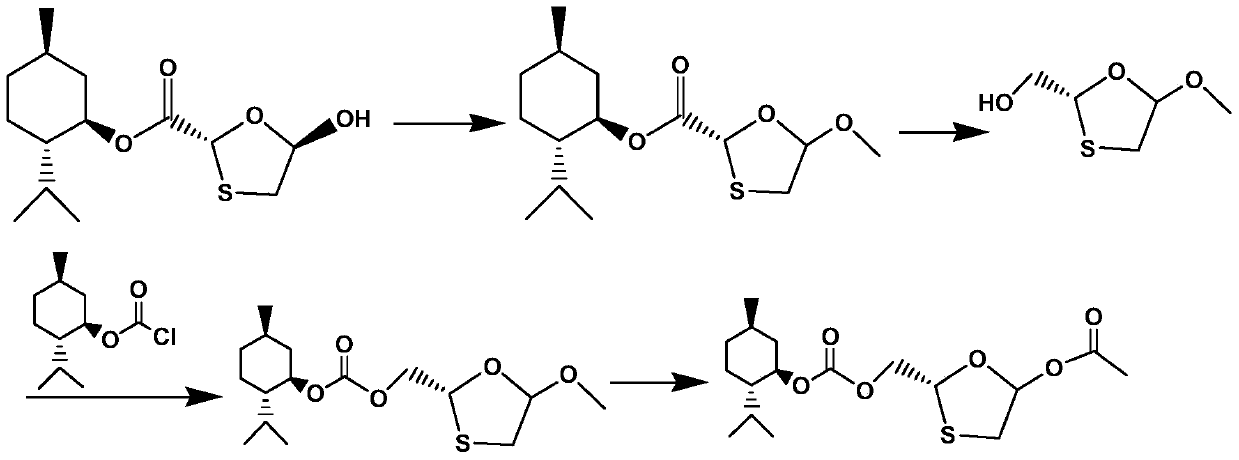
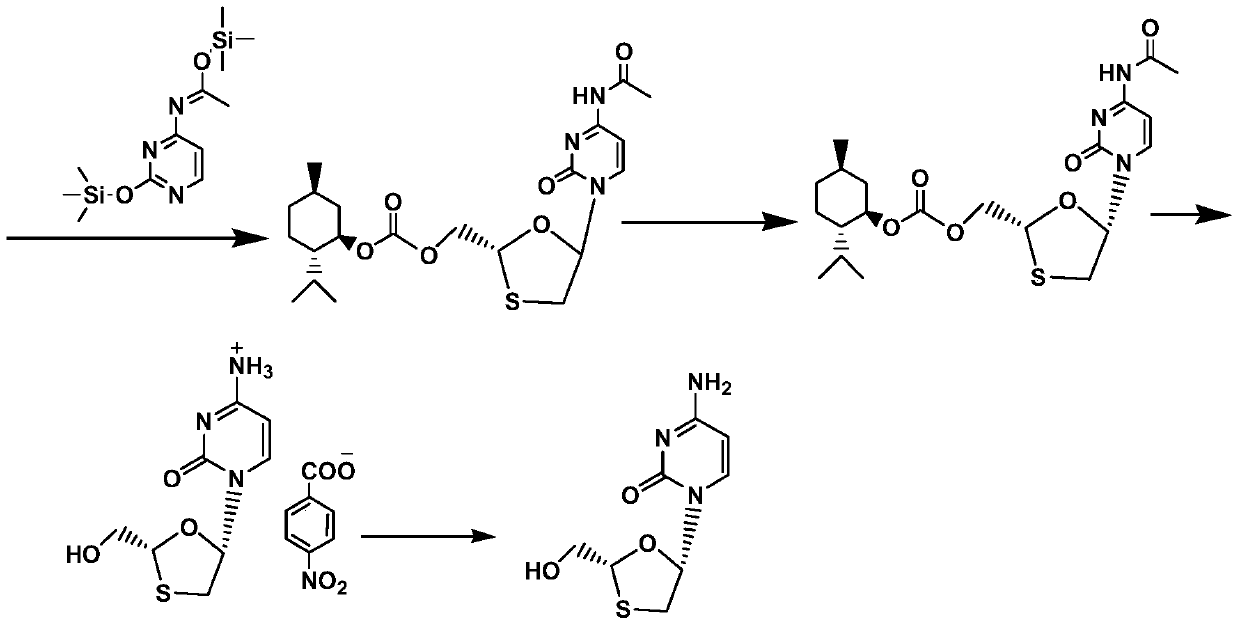
A kind of method of synthesizing lamivudine intermediate
A technology of lamivudine and an intermediate, which is applied in the field of pharmaceutical synthesis, can solve the problems of difficult separation of enantiomers, easy generation of a large number of degraded impurities, easy generation of emulsification, etc., and achieves simple post-processing operations, improved separation efficiency, The effect of saving the cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1kg of compound shown in formula VI was added to 1vol% hydrochloric acid methanol solution, and reacted at 20~25°C for 2~2.5 hours; after the reaction, 0.05kg of potassium carbonate was added to the reaction flask, and then the pH value was adjusted to 7 with sodium carbonate. ~8; filter with suction and concentrate at 40-45°C to obtain the compound represented by formula V (oily substance), which is ready for use.
[0036] Add 1.2kg95vol% ethanol and 0.2kg sodium borohydride to the 5L reaction flask; after the compound shown in the oily formula V obtained in the previous step is dissolved with 0.8kg95vol% ethanol, it is added dropwise to the sodium borohydride ethanol solution at 20~25°C , the dripping rate is controlled to be dripped within 4.5 to 5 hours; after dripping, the temperature is raised to 38 to 42 ° C, and the reaction is kept stirring for about 2 hours, and the reaction is monitored by TLC; ~6.5; then extract with dichloromethane, collect the organic phas...
Embodiment 2
[0041]Take 50g of the meso compound shown in the above-mentioned formula I', add 300g of ethanol, heat to reflux and dissolve under stirring, then slowly drip 500g of n-hexane under the reflux state, dripping is completed, and cool to 0~5 ℃, Incubate and stir for 1 hour, filter, wash, collect the filter cake, vacuum-dry at 50 ° C for 5 hours, the obtained 21.2g solid is the lamivudine intermediate shown in formula I, the mass yield is 42.4%, and the HPLC purity is 98.5%, of which the content of diastereomers is 0.86%.
Embodiment 3
[0043] Get 50g meso compound shown in above-mentioned formula I ', add 200g n-butanol, under stirring, heat to reflux and dissolve clear, then slowly drip 450g n-hexane under reflux state, drip complete, cool down to 0~5 ℃, keep stirring for 1 hour, filter, wash, collect filter cake, vacuum-dry at 50 ℃ for 5 hours, the obtained 24.3g solid is the lamivudine intermediate shown in formula I, mass yield is 48.6%, HPLC The purity was 98.9%, and the content of diastereomers was 0.35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com