Catalytic Synthesis of Oxalic Acid Combined Process of Batch Reaction and Continuous Reaction Rectification
A technology of reactive distillation and combined process, applied in the chemical industry, sustainable manufacturing/processing, separation/purification of carboxylic acid compounds, etc., can solve the problems of complicated purification and separation of subsequent products, long reaction time, low conversion rate, etc. , to achieve the effect of saving energy consumption and investment, reducing energy consumption, and reducing the ratio of water to ester
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
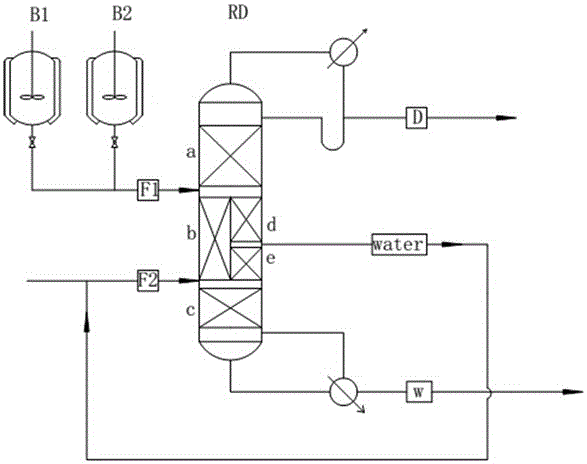
[0029] Water and dimethyl oxalate are mixed at a molar ratio of 5:1, enter the batch reactor, and carry out preliminary reaction at 65℃. After 3 hours of reaction, feed to the next-wall rectification tower. The reaction liquid in the batch reactor is fed from the upper inlet Continuously enter the reaction zone (zone b) of the next-wall tower RD, and the corresponding ratio (total water-to-ester ratio of the entire process is 10:1) of room temperature water enters the tower from the lower feed port of the reaction zone (zone b), and the top of the tower will react The methanol produced in the reactor is continuously discharged, part of the water is continuously produced in the lower part of the rectification zone (zone d) on the right side of the next wall tower, and oxalic acid, excess water and a very small amount of methanol are continuously produced in the tower bottom. The specific parameters are as follows:
[0030] (1) The volume of the batch reactor is 10m 3 The reaction t...
Embodiment 2
[0037] Water and dimethyl oxalate are mixed with a molar ratio of 4:1, enter the intermittent reactor, and carry out preliminary reaction at 60℃. After about 4 hours, feed to the next rectification tower. The reaction liquid in the intermittent reactor is fed from the upper inlet Continuously enter the reaction zone (zone b) of the next-wall tower RD, and the corresponding ratio (total water to ester ratio of the whole process is 10:1) of room temperature water enters the tower from the lower feed port of the reaction zone (zone b), and the top of the tower will react The methanol produced in the reactor is continuously discharged, part of the water is continuously produced in the lower part of the rectification zone (zone d) on the right side of the next wall tower, and oxalic acid, excess water and a very small amount of methanol are continuously produced in the tower bottom. The specific parameters are as follows:
[0038] (1) The volume of the batch reactor is 10m 3 The reacti...
Embodiment 3
[0045] Water and dimethyl oxalate are mixed at a molar ratio of 5:1, enter the batch reactor, and carry out preliminary reaction at 65℃. After 3 hours of reaction, feed to the next-wall rectification tower. The reaction liquid in the batch reactor is fed from the upper inlet Continuously enter the reaction zone (zone b) of the next-wall tower RD, and the corresponding ratio (total water to ester ratio of the whole process is 11:1) of room temperature water enters the tower from the lower feed port of the reaction zone (zone b), and the top of the tower will react The methanol produced in the reactor is continuously discharged, part of the water is continuously produced in the lower part of the rectification zone (zone d) on the right side of the next wall tower, and oxalic acid, excess water and a very small amount of methanol are continuously produced in the tower bottom. The specific parameters are as follows:
[0046] (1) The volume of the batch reactor is 10m 3 The reaction te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com