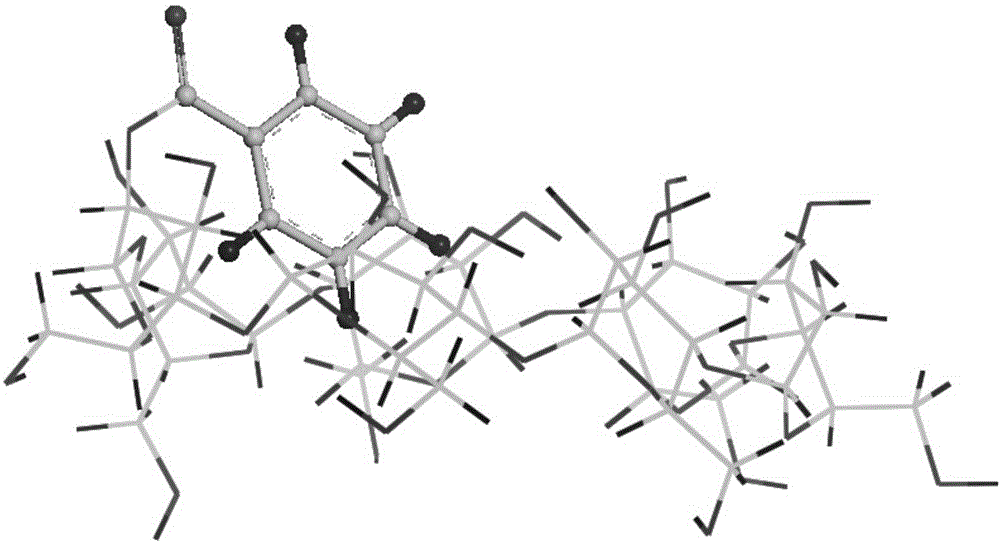
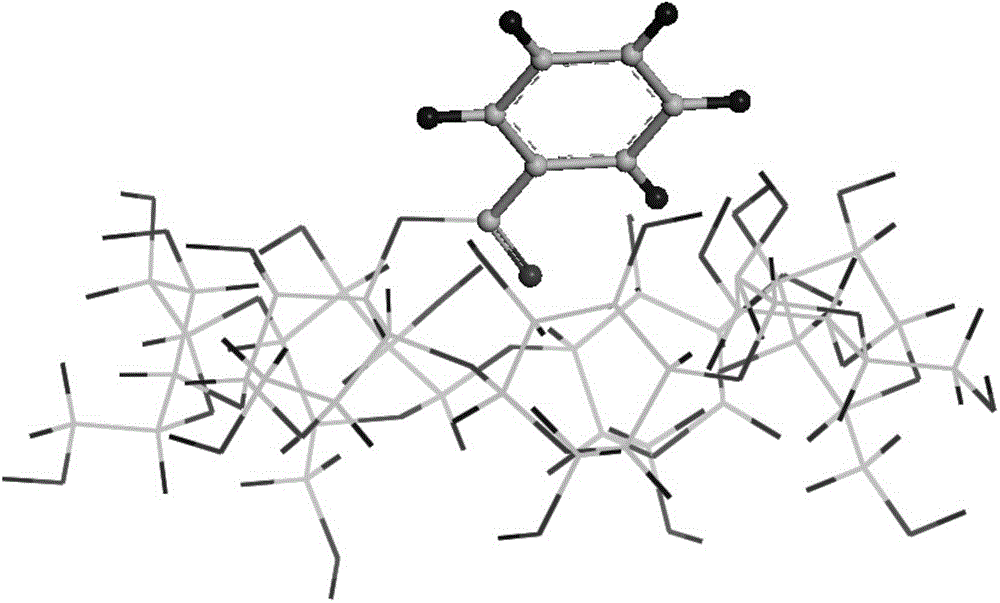
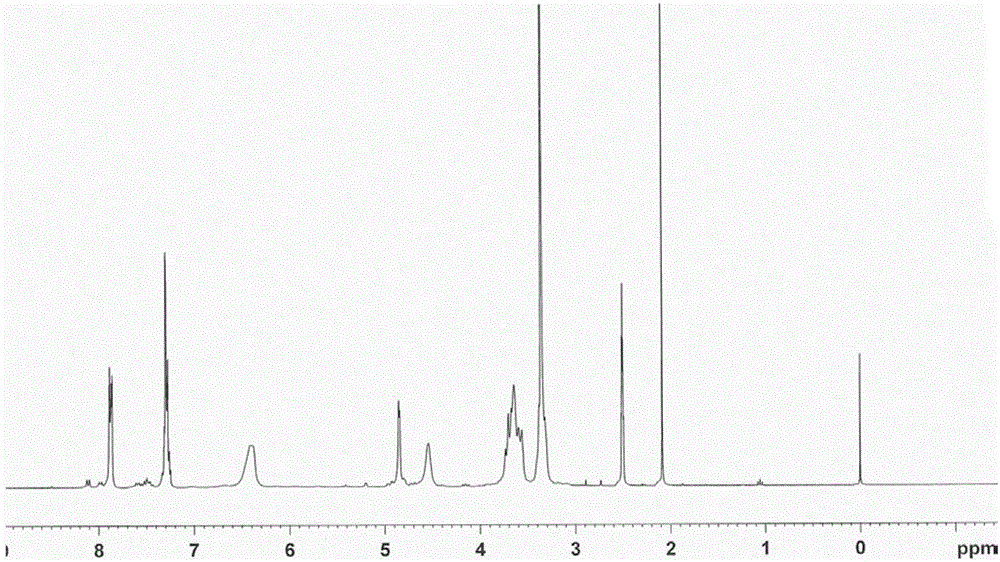
Preparation method of 3-site substituted benzoyl-beta-cyclodextrin
A technology of benzoyl and cyclodextrin, which is applied in the field of preparation of benzoyl-β-cyclodextrin, can solve the problems of many by-products and low yield, and achieve the effect of strong operability and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of 3-substituted benzoyl-β-cyclodextrin, comprising steps as follows:
[0046] (1) Dissolve 6.63g (48mmol) of potassium carbonate and 6.81g (6.0mmol) of β-cyclodextrin in a mixed solvent consisting of 40mL of purified water and 15mL of N,N-dimethylformamide (DMF). solution;
[0047](2) Dissolve 6.75g (48mmol) of benzoyl chloride in 25mL of N,N-dimethylformamide (DMF), and add it dropwise to the mixed solution obtained in step (1) within 1h at 20°C, Add 3mol / L potassium hydroxide solution during the dropwise addition to maintain the pH of the solution between 9 and 11;
[0048] (3) After the dropwise addition is completed, neutralize the solution obtained in step (2) with 2mol / L hydrochloric acid to neutrality;
[0049] (4) Distill under reduced pressure at 75mmHg and 60°C, evaporate the solution obtained in step (3) to dryness, and vacuum-dry at 40°C for 6 hours to obtain a crude product;
[0050] (5) Add 10 g of the crude product obtained in ste...
Embodiment 2
[0062] A preparation method of 3-substituted benzoyl-β-cyclodextrin, comprising steps as follows:
[0063] (1) Dissolve 1g of potassium carbonate and 10g of β-cyclodextrin in a mixed solvent consisting of 20mL of N,N-dimethylformamide (DMF) and 50mL of purified water to obtain a mixed solution;
[0064] (2) Dissolve 10g of benzoyl chloride in 25mL of N,N-dimethylformamide (DMF), and add it dropwise to the mixed solution obtained in step (1) within 5 hours at 10°C. 3mol / L potassium hydroxide solution to maintain the pH of the solution between 9 and 11;
[0065] (3) After the dropwise addition is completed, neutralize the solution obtained in step (2) with 2mol / L hydrochloric acid to neutrality;
[0066] (4) Distill under reduced pressure at 75mmHg and 60°C, evaporate the solution obtained in step (3) to dryness, and vacuum-dry at 60°C for 4 hours to obtain a crude product;
[0067] (5) Add 15 g of the crude product obtained in step (4) into 50 mL of N,N-dimethylformamide (DMF...
Embodiment 3
[0071] A preparation method of 3-substituted benzoyl-β-cyclodextrin, comprising steps as follows:
[0072] (1) Dissolve 10 g of potassium carbonate and 1 g of β-cyclodextrin in a mixed solvent consisting of 20 mL of N,N-dimethylformamide (DMF) and 50 mL of purified water to obtain a mixed solution;
[0073] (2) Dissolve 10g of benzoyl chloride in 25mL of N,N-dimethylformamide (DMF), and add it dropwise to the mixed solution obtained in step (1) within 1 hour at 80°C. 3mol / L potassium hydroxide solution to maintain the pH of the solution between 9 and 11;
[0074] (3) After the dropwise addition is completed, neutralize the solution obtained in step (2) with 2mol / L hydrochloric acid to neutrality;
[0075] (4) Distill under reduced pressure at 75mmHg and 60°C, evaporate the solution obtained in step (3) to dryness, and vacuum-dry at 20°C for 6 hours to obtain a crude product;
[0076] (5) Add 6 g of the crude product obtained in step (4) into 50 mL of N,N-dimethylformamide (D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com