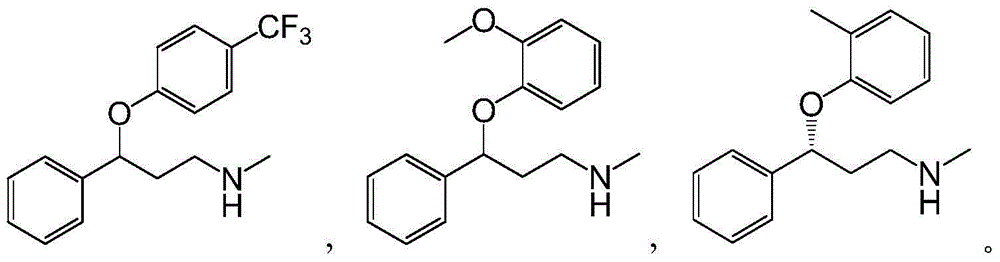
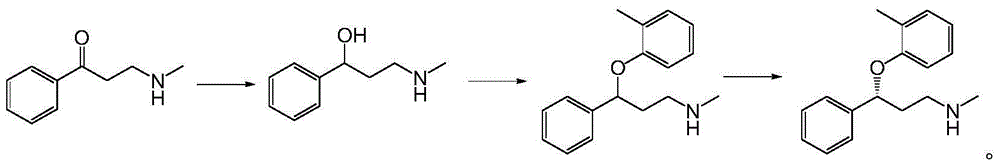
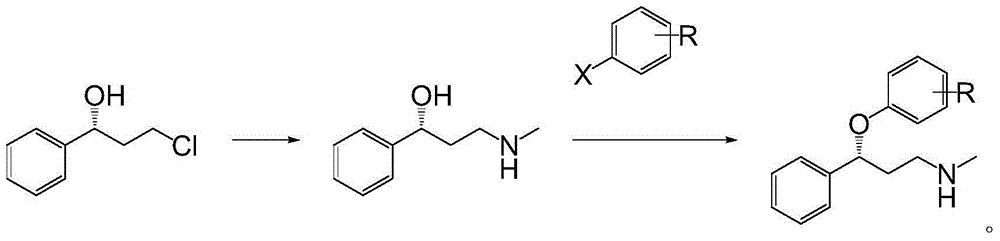
Applications of alcohol dehydrogenase in asymmetric reduction
An alcohol dehydrogenase, asymmetric technology, applied in atomoxetine, the field of preparation of single-configuration oxetine drugs, can solve the problems of unfriendly environment, low reaction yield, unsuitable for large-scale industrial production and the like, To achieve the effects of environmental friendliness, high product concentration, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Dissolve 163g of 3-(methylamino)-1-phenylpropan-1-one in 150mL of isopropanol, add 400mL of sodium phosphate buffer and adjust the pH to 7.0 with 20% (w / v) NaOH solution, add 450mL of the whole bacteria lysate was stirred at 30°C for reaction, and the reaction progress was detected by TLC. After the reaction, dilute hydrochloric acid was added to adjust the pH to 2 to precipitate the protein, and then filtered with diatomaceous earth, the filtrate was adjusted to pH 12 with 20% (w / v) sodium hydroxide solution, extracted three times with equal volume of ethyl acetate, The organic phases were combined, dried and spin-dried to obtain the product. The weight of the product is 150.1 g, and the molar yield is 91.0%.
[0043] The structure of the product was confirmed by proton nuclear magnetic resonance spectroscopy and ee value determination:
[0044]
[0045] 1 H NMR (300MHz, CDCl 3 ):δ7.19(5H,s,Ar-H),4.50(1H,t,-CHOH),2.55(2H,t,-CH 2 NHCH 3 ),2.47(3H,s,-CH3 ),2.0(1...
Embodiment 2-5
[0048] Referring to the method of Example 1, in combination with the parameters in Table 1, the compounds of Examples 2-5 were prepared. Wherein, when NADP+ is used in the reaction system, NADP+ and the whole bacterial lysate are added to the reaction system at the same time.
[0049] Table 1
[0050]
[0051]
[0052] The detection and structural characterization results of the product are as follows.
Embodiment 2
[0054]
[0055] The weight of the product is 92.0 g, and the molar yield is 91.0%.
[0056] 1 H NMR (300MHz, CDCl3): δ7.19(5H, s, Ar-H), 4.50(1H, t, -CH(OH)), 2.36(2H, t, -CH 2 NMe 2 ),2.27(6H,s,-N(CH 3 ) 2 ),2.0(1H,s,-OH).1.87(2H,m,-C(OH)CH 2 -),
[0057] ee value >99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com