Mark product of <89>Zr marked denosumab, and preparation method and application thereof
A technology of denosumab and 89zr, which is applied in the direction of radioactive carriers, can solve the problems of inaccurate imaging results, objectivity, low radiochemical purity, and the inability to achieve long-term experiments and inspections, and achieve imaging results Objective, radiochemically pure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
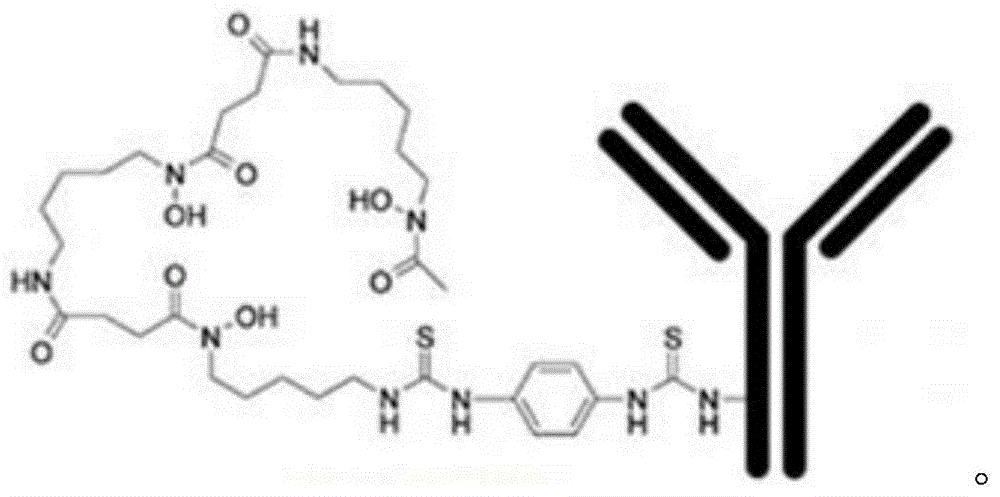
[0034] In this example, the used 89 Zr oxalic acid solution was purchased from PerkinElmer. The Df-Bz-NCS-denosumab used was obtained by:
[0035] Take NaHCO with a concentration of 0.1mol / L and a pH value of 8.4 3 Dissolve denosumab in the solution so that the concentration of denosumab is 10mg / ml, and then use 0.1mol / L Na 2 CO 3 Solution is adjusted to its pH value to 9.0; Then add bifunctional chelating agent ferric isothiocyanate (abbreviation " BFC (Df-Bz-NCS) " wherein, namely hereinafter the same) concentration of 2mmol / L dimethyl sulfoxide (abbreviated as DMSO, hereinafter the same) solution, so that the molar ratio of bifunctional chelating agent and denosumab is 5:1, after mixing, react at 37 ℃ for 60min, namely Obtain Df-Bz-NCS-denosumab crude product; The obtained Df-Bz-NCS-denosumab crude product is purified by PD10 column, after purification, adding physiological saline and gentisic acid solution with a concentration of 3.5 mg / ml as a stabilizer, Store at -...
Embodiment 2
[0049] In this embodiment, adopt the same method as in Embodiment 1 89 Zr oxalic acid solution. The Df-Bz-NCS-denosumab used is obtained in the following way: taking NaHCO with a concentration of 0.1mol / L and a pH value of 8.4 3 Dissolve denosumab in the solution so that the concentration of denosumab is 10mg / ml, and then use 0.1mol / L Na 2 CO 3 The solution adjusts its pH value to 8.9; Then the concentration of BFC (Df-Bz-NCS) added thereto is the DMSO solution of 2mmol / L, makes the mol ratio of bifunctional chelating agent and denosumab be 5:1, after mixing, at 37 The crude product of Df-Bz-NCS-denosumab was obtained by reacting at ℃ for 60 minutes; the obtained crude product of Df-Bz-NCS-denosumab was purified by PD10 column, after purification, physiological saline and gentisic acid solution with a concentration of 3.5mg / ml were added As a stabilizer, store at -80°C until use.
[0050] In this embodiment 89 The method for Zr marking denosumab specifically comprises the...
Embodiment 3
[0060] In this embodiment, the same Df-Bz-NCS-denosumab and 89 Zr oxalic acid solution.
[0061] In this embodiment 89 The method for Zr marking denosumab specifically comprises the following steps:
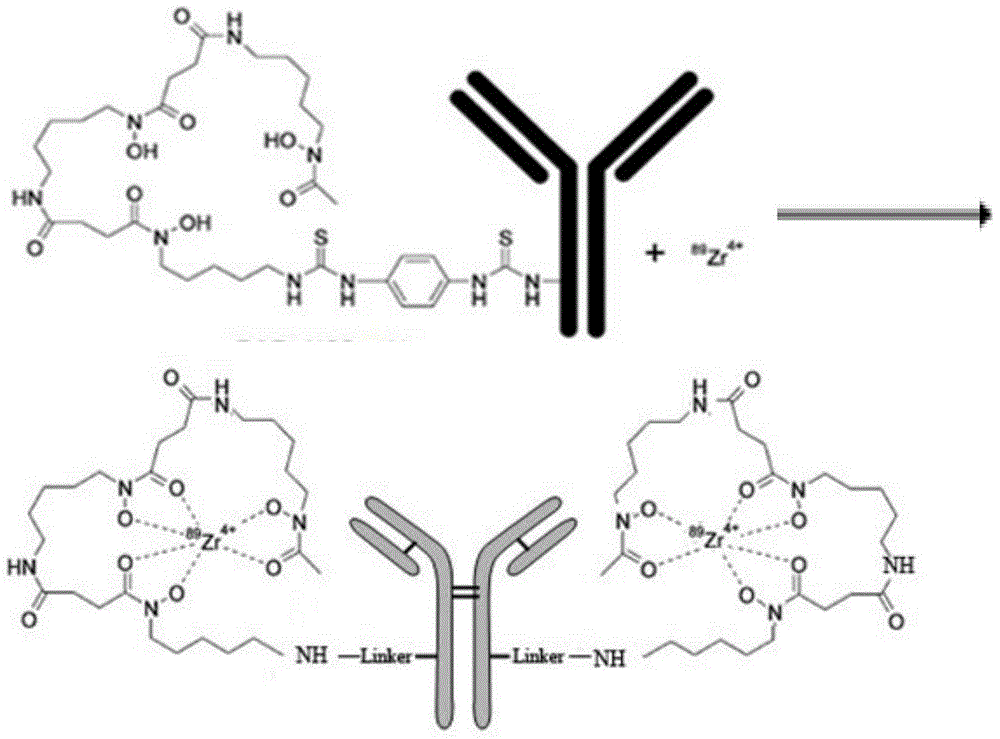
[0062] Take 100 μl of 4 mCi radioactivity 89 Zr oxalic acid solution, with the concentration being 0.1mol / L, the sodium carbonate solution that pH value is 10 and described 89 Zr oxalic acid solution to a pH value of 7.2, then, adding 0.3ml of sodium edetate buffer solution with a concentration of 0.1mol / L and a pH value of 6.8, then adding Df-Bz-NCS-denosumab therein, and mixing evenly, The reaction solution was formed and reacted at 25°C for 40 minutes to obtain the labeled product [ 89 Zr]-Df-Bz-NCS-denosumab.
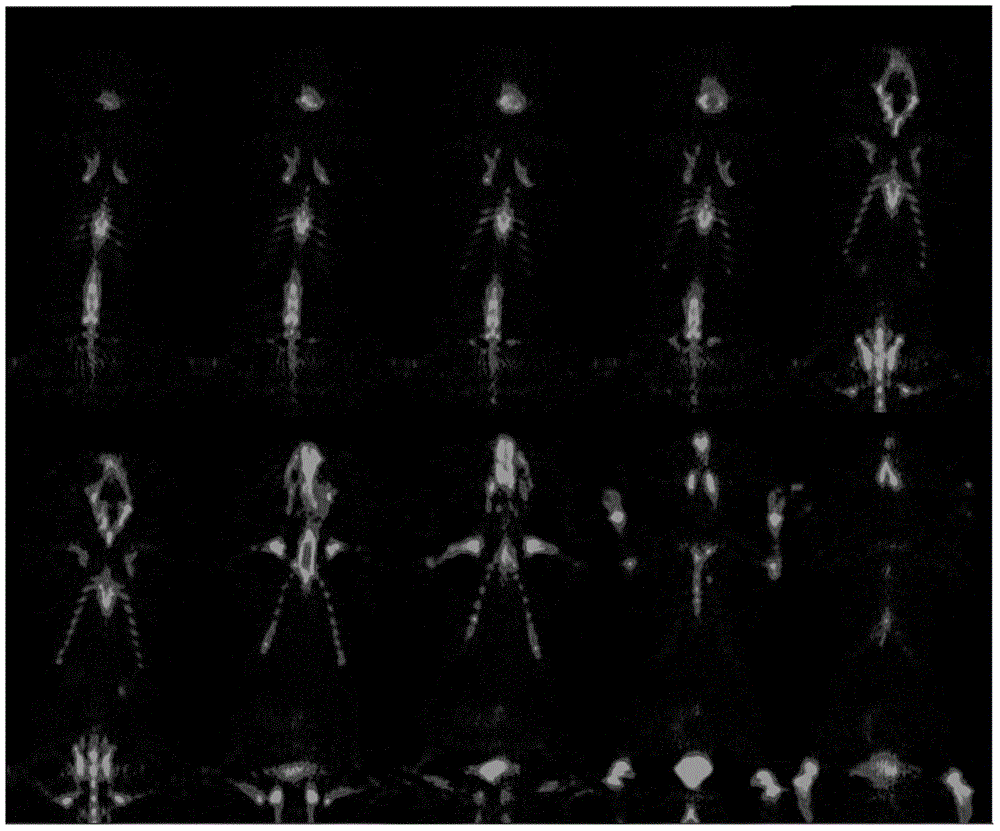
[0063] Using the method in Example 1 to obtain the labeled product [ 89 The stability and radiochemical purity of Zr]-Df-Bz-NCS-denosumab were measured, and the results were as follows:
[0064] (1) iTLC method for measuring radiochemical purity: calculate [ 89...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com