A kind of preparation method of 2-chloro-5-ethylpyridine
A technology of ethylpyridine and vinylpyridine, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problems of low yield and long steps of key ring-forming steps , to achieve the effect of convenient purification, high selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0027] The preparation of embodiment 12-chloro-5-vinylpyridine (method 1)
[0028] In a 500ml pressure bottle, mix 2-chloro-5-bromopyridine (9.6g, 50mmol) and 4,4,5,5-tetramethyl-2-vinyl-1,3,2-dioxaborol Naphthene (10.0 g, 65 mmol) was added to ethylene glycol dimethyl ether (225 mL) and 2M aqueous sodium carbonate (75 mL) to form a two-phase mixed system. Under nitrogen atmosphere, tetrakis(triphenylphosphine)palladium(0) (2.9 g, 2.5 mmol) was added to the mixed system. Subsequently, the pressure bottle was sealed, and the reaction system was stirred at 84° C. for 16 hours. The reaction mixture was diluted with 150 mL of water and 150 mL of ethyl acetate, and the white solid was removed by filtration. After the two phases were separated, the aqueous phase was extracted with 40 ml of ethyl acetate, the combined organic phase was dried over anhydrous magnesium sulfate, filtered, concentrated by rotary evaporation to about 20 ml, and purified by column chromatography to obtain...
Embodiment 22
[0030] The preparation of embodiment 22-chloro-5-vinylpyridine (method 2)
[0031] Potassium tert-butoxide (28 g, 250 mmol) was added portionwise to a suspension of methyltriphenylphosphine bromide (107 g, 300 mmol) in anhydrous tetrahydrofuran (500 ml) at 0°C. After stirring for 0.5 hour, a solution of 2-chloro-5-formylpyridine (35.4 g, 250 mmol) in anhydrous tetrahydrofuran (200 ml) was slowly added dropwise to the reaction system. After the dropwise addition was complete, the reaction mixture was stirred at 0° C. for 1 hour. Subsequently, the ice bath was removed, and the reaction mixture was stirred at room temperature for 1.5 hours. Saturated ammonium chloride solution (750ml) and ethyl acetate (400ml) were added. After the two phases were separated, the aqueous phase was extracted with 100 ml of ethyl acetate, the combined organic phase was dried over anhydrous magnesium sulfate, filtered, concentrated by rotary evaporation to about 100 ml, and purified by column chrom...
Embodiment 32
[0032] The preparation of embodiment 32-chloro-5-ethylpyridine
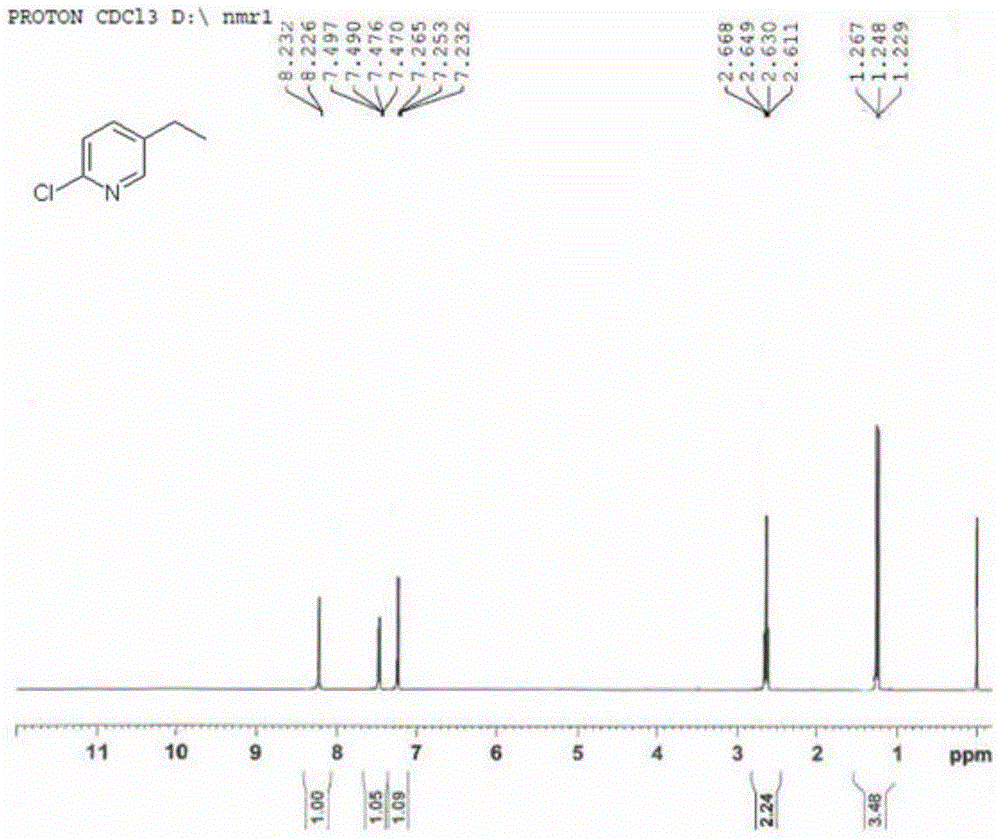
[0033] 2-Chloro-5-vinylpyridine (1g, 7.2mmol) was dissolved in 20ml of methanol, and after the resulting solution was passed through hydrogen for 2 minutes, 1,5-cyclooctadiene (pyridine) (tricyclohexylphosphine) iridium (I ) hexafluorophosphate catalyst (0.1 g), and subsequently, the mixture was reacted in a hydrogenation reactor (1 atm of hydrogen) for 2 hours. After the reaction solution was filtered and concentrated through diatomaceous earth, 10ml of dichloromethane and 10ml of water were added. After the organic phase was separated, it was dried with anhydrous magnesium sulfate, filtered, and concentrated by rotary evaporation to obtain 0.84g of 2-chloro-5-ethylpyridine in the form of Pale yellow liquid, yield 83%. The purity detected by HPLC is greater than 98%.
[0034] MS(EI)m / z 142.3[MH] + ; 1 H-NMR (400MHz, CDCl 3 ) δppm: 8.23(d, 1H), 7.48(q, 1H), 7.25(t, 1H), 2.63(q, 2H), 1.25(t, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com