Manganese vanadate nano-micro material, and synthetic method and application thereof
A technology of manganese vanadate nano and manganese vanadate, which is applied in nanotechnology, manganese compounds, nanotechnology, etc., to achieve the effect of excellent product crystallization performance, easy control of morphology and structure, and suitable for mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
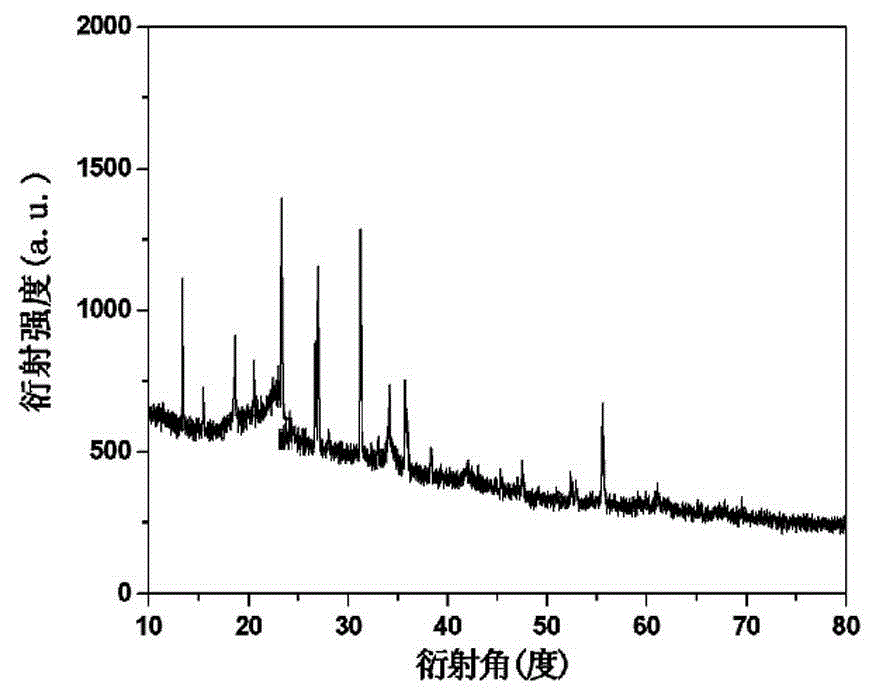
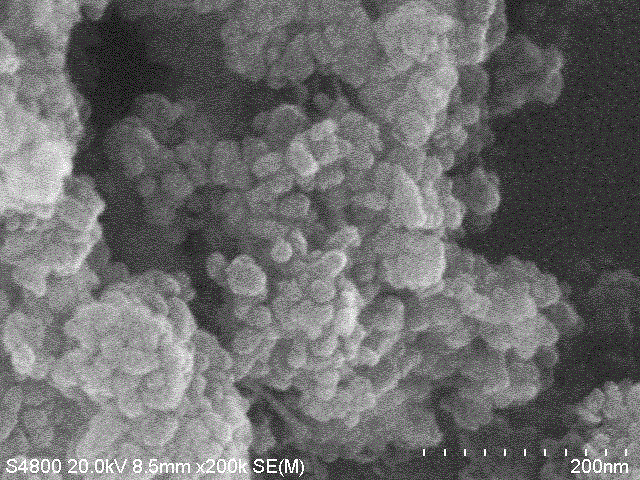
[0022] At room temperature, dissolve 2mmol of sodium orthovanadate in 6mL of distilled water, and add 1mmol of MnSO 4 ·H 2 Dissolve O in 8 mL of distilled water, add the sodium orthovanadate solution dropwise to MnSO under magnetic stirring 4 In the solution, stir for 10 min, with 1mol / L of H 2 SO 4 The solution adjusts the pH value of the reaction system to 7, continues to stir for 10 minutes, then transfers it into a 20 mL hydrothermal reactor, seals it and places it in a thermostat for hydrothermal reaction at 160 ℃ for 6 hours, and cools to room temperature after the reaction is complete , Washed with deionized water and absolute ethanol for 3 times, centrifuged, and put the resulting precipitate in an oven at 60 ℃ for vacuum drying for 16 hours to obtain manganese vanadate Mn 6.87 (OH) 3 (VO 4 ) 3.6 (V 2 O 7 ) 0.2 material. The above products were analyzed by X-ray diffraction (XRD) and scanning electron microscopy, and the results were as follows: figure 1 , figure 2 As sh...
Embodiment 2
[0024] At room temperature, dissolve 0.2mmol of sodium orthovanadate in 6 mL of distilled water, and add 0.1mmol of MnCl 2 Dissolve in 8mL distilled water, under the action of magnetic stirring, add sodium orthovanadate solution dropwise to MnCl 2 In the solution, stir for 10 minutes, adjust the pH value of the reaction system to 8 with 1mol / L HCl solution, continue to stir for 10 minutes, then transfer to a 20 mL hydrothermal reactor, seal it and place it in a thermostat Hydrothermal reaction at 180 ℃ for 4h, cooling to room temperature after the completion of the reaction, washing with deionized water and absolute ethanol for 5 times, centrifugation, and placing the obtained precipitate in an oven at 80 ℃ for vacuum drying for 8 hours to obtain manganese vanadate Mn 6.87 (OH) 3 (VO 4 ) 3.6 (V 2 O 7 ) 0.2 Nanoparticle materials are spherical particles with a diameter of 30-60nm.
Embodiment 3
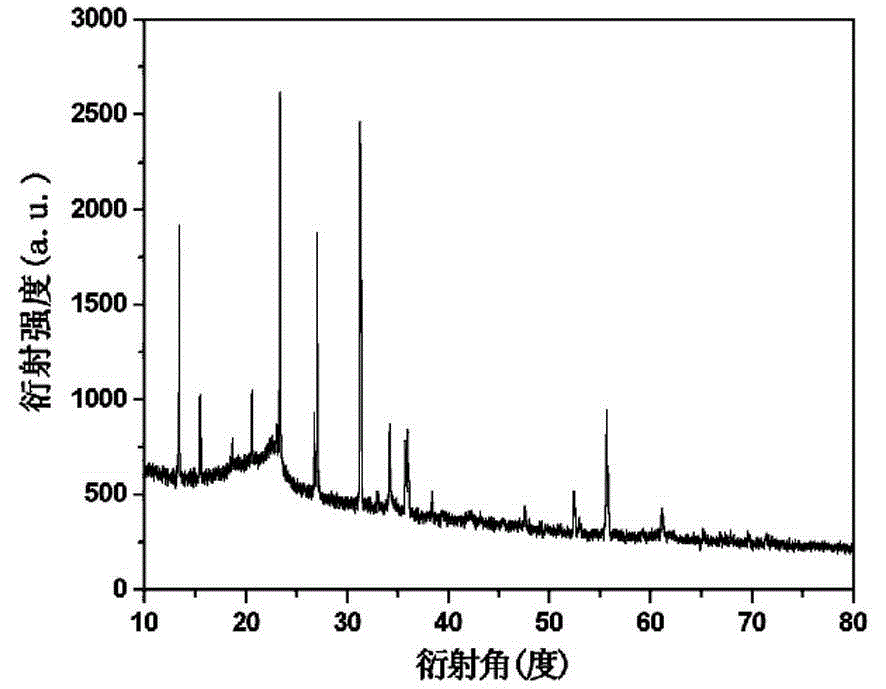
[0026] At room temperature, dissolve 2mmol of sodium orthovanadate in 6mL of distilled water, and add 2mmol of MnCl 2 Dissolve in 8 mL of distilled water, add sodium orthovanadate solution dropwise to MnCl under magnetic stirring 2 In the solution, stir for 10 min, with 1mol / L HNO 3 The solution adjusts the pH value of the reaction system to 8 and continues to stir for 10 minutes, then transfer it into a 20 mL hydrothermal reactor, seal it and place it in a thermostat for hydrothermal reaction at 180 ℃ for 22 h, and cool to At room temperature, wash with deionized water and absolute ethanol for 5 times, centrifuge, and place the obtained precipitate in an oven at 80 ℃ for vacuum drying for 8 hours to obtain manganese vanadate Mn 6.87 (OH) 3 (VO 4 ) 3.6 (V 2 O 7 ) 0.2 material. The above products were analyzed by XRD and scanning electron microscopy, and the results were as follows: image 3 , Figure 4 As shown, the XRD spectrum results show that the intensity and position of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com