Use of deferasirox in preparation of medicine for treating postmenopausal osteoporosis diseases
A technology for defrost and osteoporosis, which is applied in the field of medicine to achieve the effects of high safety, low toxicity, and reduction of bone loss.
Inactive Publication Date: 2015-04-22
徐又佳
View PDF0 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, when the iron ions in the blood exceed the binding capacity of transferrin, free iron that is not bound by transferrin will be formed, resulting in iron overload
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment
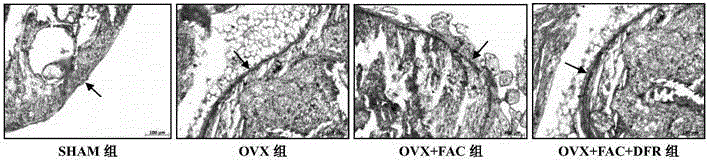
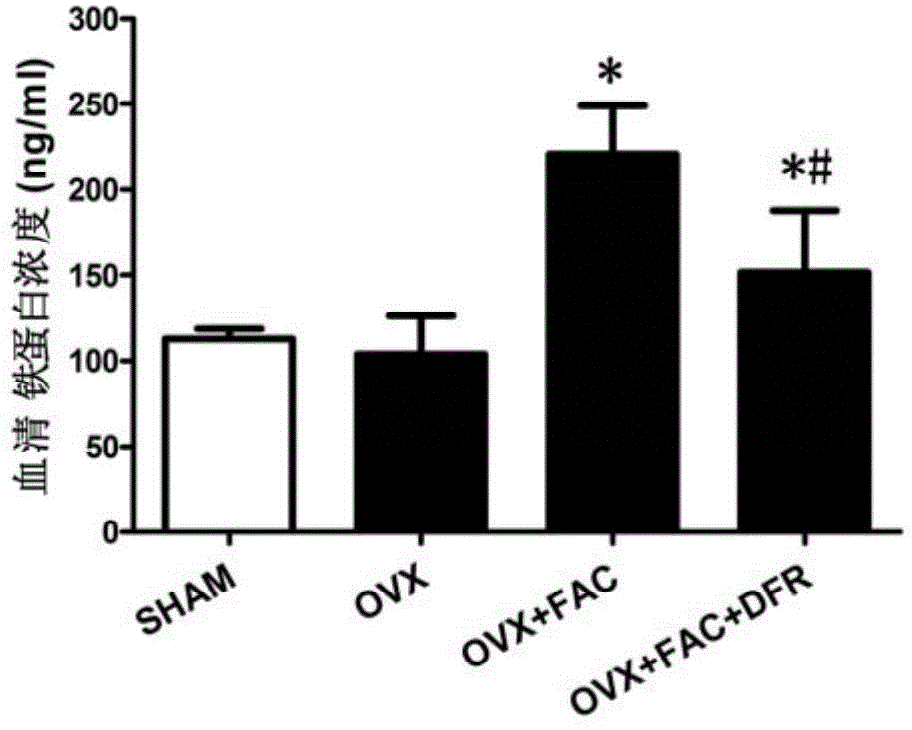
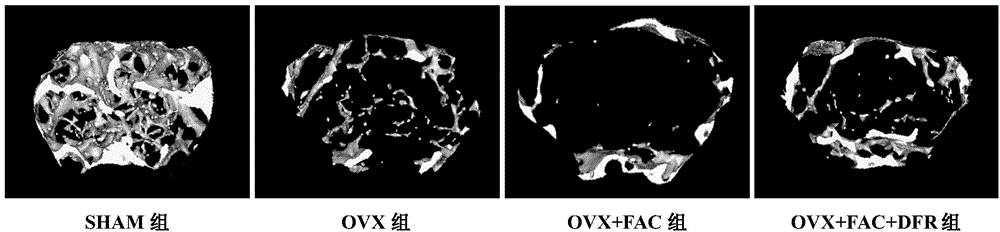
[0020] Embodiment: According to the animal experiment results of deferasirox in treating postmenopausal osteoporosis, and the clinical application method of deferasirox, in the present invention, deferasirox can be taken orally for postmenopausal osteoporosis, and each time 40mg / kg, 5 times a week.
[0021] The research findings supporting this experiment are as follows:
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention mainly relates to use of deferasirox, and in particular relates to use of deferasirox in preparation of a medicine for treating postmenopausal osteoporosis diseases. Deferasirox is used as the first oral iron chelating agent; compared with the traditional subcutaneous injection iron chelating agent, the deferasirox is low in toxicity, high in safety, convenient to administrate and good in compliance; and loss of postmenopausal bone mass can be obviously reduced.
Description
technical field [0001] The invention relates to the technical field of medicine, in particular to the use of defesirox, in particular to the use of defesirox in the preparation of medicines for treating postmenopausal osteoporosis. Background technique [0002] Deferasirox (DFR), also known as deferasirox, its chemical name is 4-[3,5-bis-(2-hydroxyphenyl)-1H-[1,2,4]-tri Azol-1-yl] benzoic acid, molecular formula is C21H15N3O4, molecular weight 373.36, and its chemical structural formula is as follows: [0003] [0004] Desferezole is an iron chelator with three protruding ligands that bind iron with high affinity in a 2:1 ratio. The half-life of defezirox in the body is about 8 to 16 hours. Its low molecular weight and high lipophilicity determine that it can be administered orally and absorbed through the intestinal tract, and then enter the cells to chelate iron, metabolize through the liver, and secrete through the bile excreted in feces. As an essential trace eleme...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/4196A61P19/10
CPCA61K31/4196
Inventor 陈斌李凯向定元汪升张鹏沈光思徐又佳
Owner 徐又佳
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com