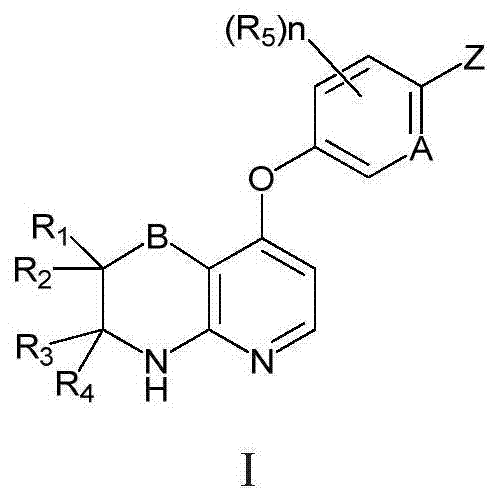
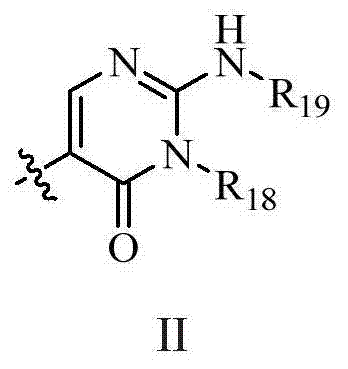
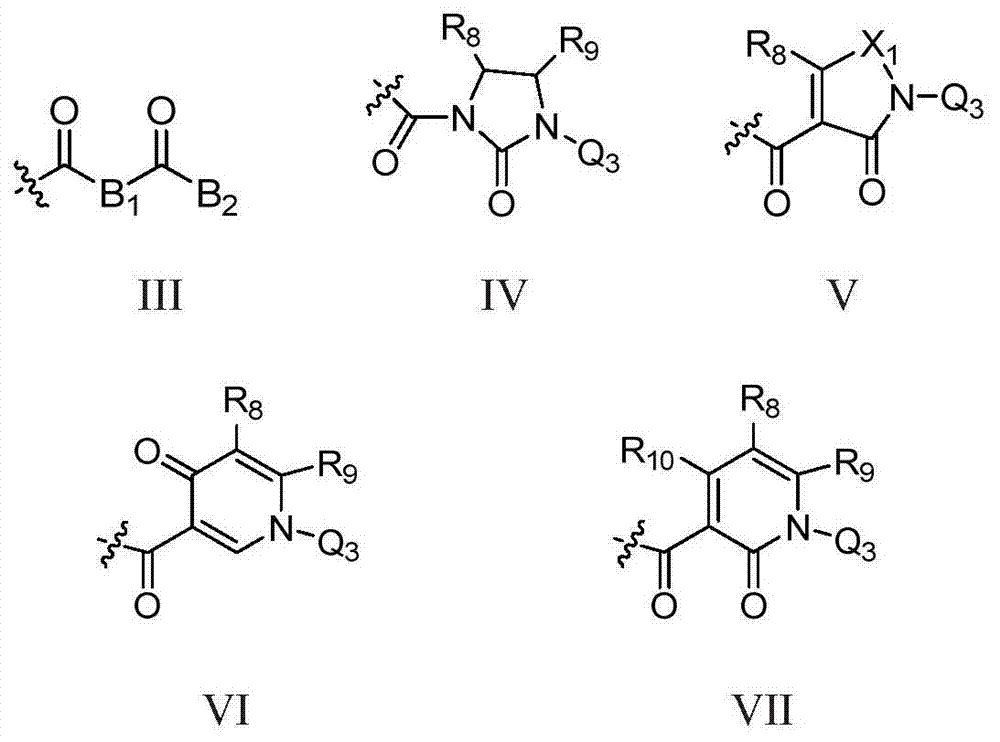
Novel fused pyridine derivatives useful as c-Met tyrosine kinase inhibitors
A kind of compound and chelate technology, applied in the field of novel fused pyridine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0382] N-(4-(2,3-dihydro-1H-pyrrolo[2,3-b]pyridine-4-oxyl)-3-fluorophenyl)-5-(4-fluorophenyl)- 4-oxo-1,4-dihydropyridine-3-carboxamide (product 1);
[0383]
[0384] Step 0. Preparation of Acid 1
[0385]
[0386] 1. Preparation of A11
[0387] To dichloromethane (450ml) was added Maxwell's acid (50.32g) followed by pyridine (70.35g). The temperature was lowered to 0° C., A10 (62.28 g) was added dropwise, and the reaction was maintained at this temperature for 2.5 hours (TLC showed that the reaction was complete). Add dichloromethane (300ml), mix and then disperse into 1N HCl (750ml), stir, place to separate, dry and concentrate the organic phase, and dry in vacuo to obtain a solid, ie A11 (66.70g).
[0388] 2. Preparation of A12
[0389] Add A11 (66.7g) into ethanol (400ml), heat to 87°C and stir overnight. The reaction mixture was cooled and the solvent was removed to give an oily substance (55 g). The obtained oily substance was dissolved in DMF / DMA (200ml), hea...
Embodiment 2
[0407] N-(3-fluoro-4-(3-oxo-3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-8-oxyl)phenyl)- 5-(4-fluorophenyl)-4-oxo-1,4-dihydropyridine-3-carboxamide (product 2);
[0408]
[0409] Step 1. Preparation of Compound 21
[0410] Compound 20 (184 mg, 1.0 mmol), 2-fluoro-4-amino-phenol (190 mg, 1.5 mmol) and cesium carbonate (652 mg, 2.0 mmol) were dissolved in N-methylpyrrolidone (3 ml). in N 2The reaction mixture was heated to 180° C. overnight. The reaction mixture was poured into water (50ml), extracted with ethyl acetate (100ml), washed with brine, dried, concentrated and purified by column chromatography to give compound 21 (105mg).
[0411] Step 2. Preparation of product 2
[0412] HATU (76 mg, 0.2 mmol), DIPEA (26 mg, 0.2 mmol), and acid 1 (25.6 mg, 0.11 mmol) were dissolved in DCM (3 ml). After stirring at 0°C for 15 minutes, compound 21 (27 mg, 0.1 mmol) was added and reacted at room temperature for 4 hours. After the reaction was completed, the reaction mixture was con...
Embodiment 3
[0414] N-(4-(3,4-dihydro-2H-pyrido[3,2-b][1,4]oxazine-8-oxyl)-3-fluorophenyl)-5-(4-fluoro Phenyl)-4-oxo-1,4-dihydropyridine-3-carboxamide (product 3);
[0415]
[0416] Step 1. Preparation of compound 31
[0417] Compound 30 (1.52g, 10mmol), 3,4-difluoronitrobenzene (1.75g, 11mmol) and cesium carbonate (3.60g, 11mmol) were dissolved in DMF (15ml). The reaction was stirred overnight at room temperature. After the reaction was completed (TLC detection), water (400ml) was added, extracted with ethyl acetate (800ml), washed with brine, dried and purified to obtain compound 31 (2.20g).
[0418] Step 2. Preparation of compound 32
[0419] Compound 31 (2.20 g) was dissolved in methanol (100 ml), and 5% Pd / C (0.8 g) was added. Access to H 2 , and the reaction was stirred to completion. Pd / C in the reaction mixture was removed by filtration, and the filtrate was concentrated to obtain compound 32 (1.60 g).
[0420] Preparation of step 3 product 3
[0421] The target product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com