N-allyl-disubstituted tetrazole, as well as preparation method and detection method thereof
An allyl and disubstituted technology, which is applied in the detection of N-allyl-disubstituted tetrazole, the preparation field of N-allyl-disubstituted tetrazole and N-allyl-disubstituted tetrazole , can solve problems such as cost increase, and achieve the effect of high total yield, mild reaction conditions, and efficient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
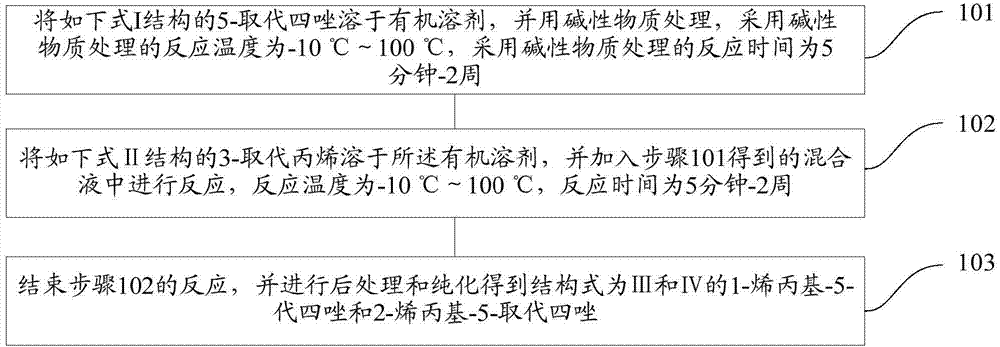
[0034] refer to figure 1 , shows a flow chart of an embodiment of a preparation method of N-allyl-disubstituted tetrazole of the present invention, which may specifically include the following steps:
[0035] Step 101, dissolving the 5-substituted tetrazole with the structure of the following formula I in an organic solvent, and treating it with an alkaline substance. The reaction temperature for the treatment with the alkaline substance is -10°C to 100°C, and the reaction time for the treatment with the alkaline substance is 5 minutes - 2 weeks.
[0036] Step 102, dissolving 3-substituted propylene with the structure of the following formula II in the organic solvent, and adding it to the mixed solution obtained in step 101 for reaction, the reaction temperature is -10°C to 100°C, and the reaction time is 5 minutes to 2 weeks .
[0037] Step 103, ending the reaction in step 102, and performing post-treatment and purification to obtain 1-allyl-5-substituted tetrazole and 2-all...
Embodiment 1
[0058] Embodiment 1: the allylation of 1,5-phenyl tetrazole, reaction formula is as follows:
[0059]
[0060] The preparation process is as follows:
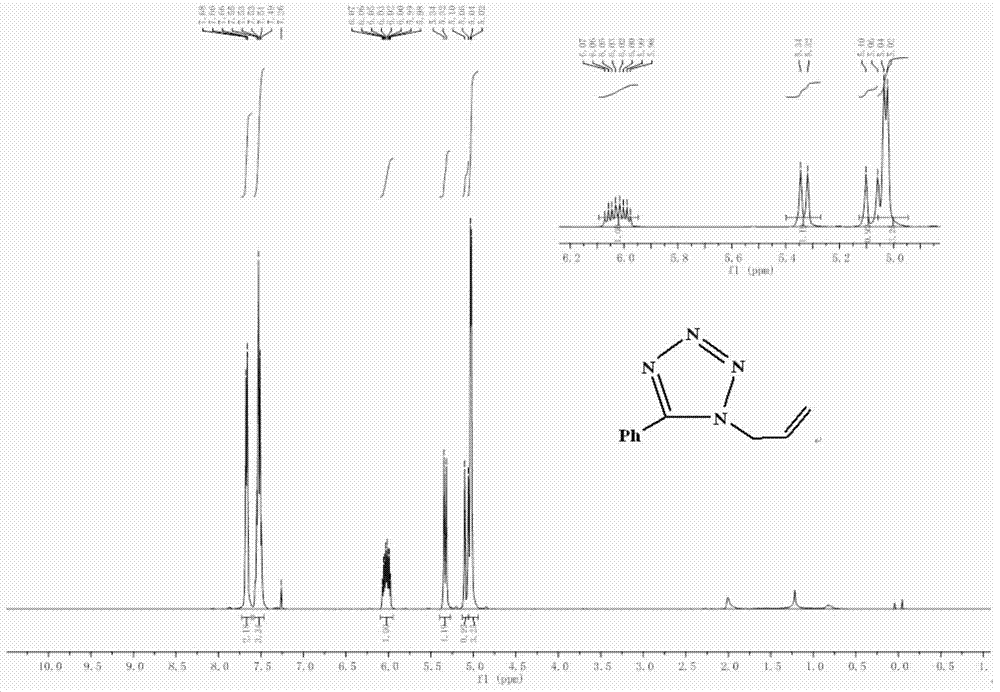
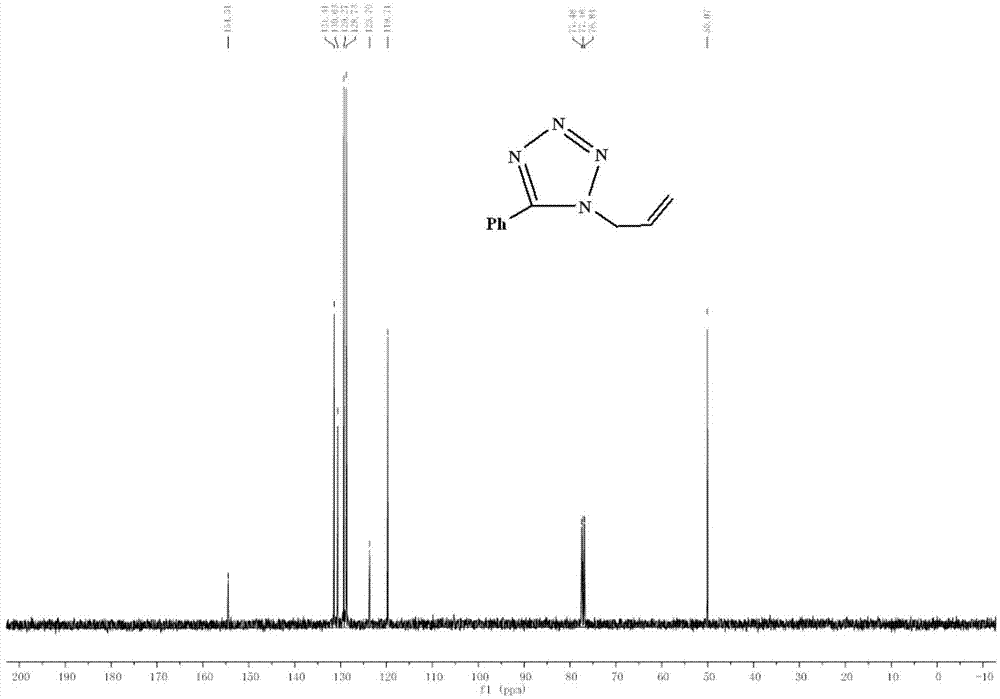
[0061] 5-Chloromethyltetrazole (485mg, 4.1mmol) and anhydrous Na 2 CO 3 (522mg, 4.92mmol) was added to acetone (10ml), stirred for 0.5h, acetone solution (10ml) containing bromopropene (992mg, 8.2mmol) was added to the above reaction mixture, and heated to 60°C. After 2.5 hours, TLC showed that the reaction was complete. Stop reaction, remove acetone, add water (30ml) to make Na 2 CO 3 Dissolve, add ethyl acetate for extraction (3×20ml), combine the organic layers, dry the organic phase with anhydrous sodium sulfate, filter and concentrate to obtain a yellow oily liquid, which is separated by column chromatography [eluent is V(EtOAc) / V(Petroleum Ether)=1 / 6], two kinds of light yellow oily liquids were obtained respectively, 1-allyl-5-phenyl tetrazole (130mg, yield 17%) and 2-allyl-5 - Phenyltetrazole (602 mg, yield 79...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com