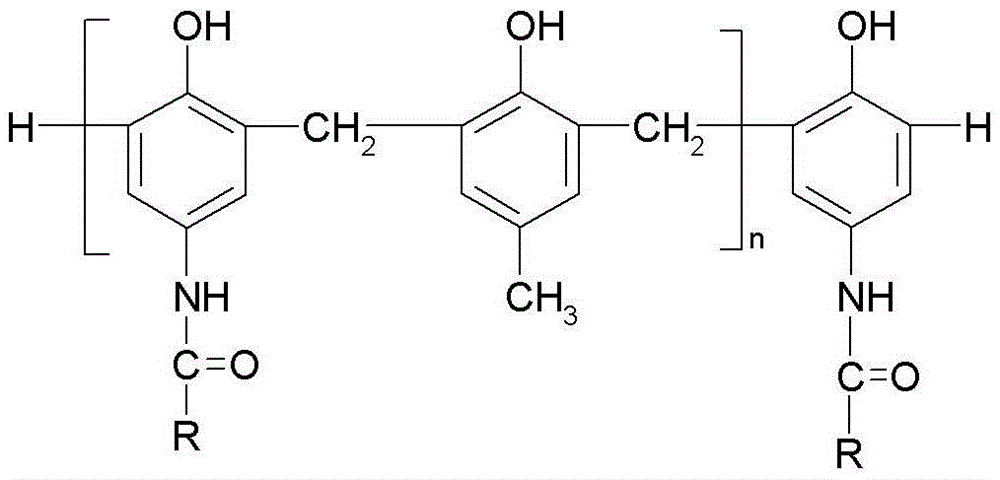
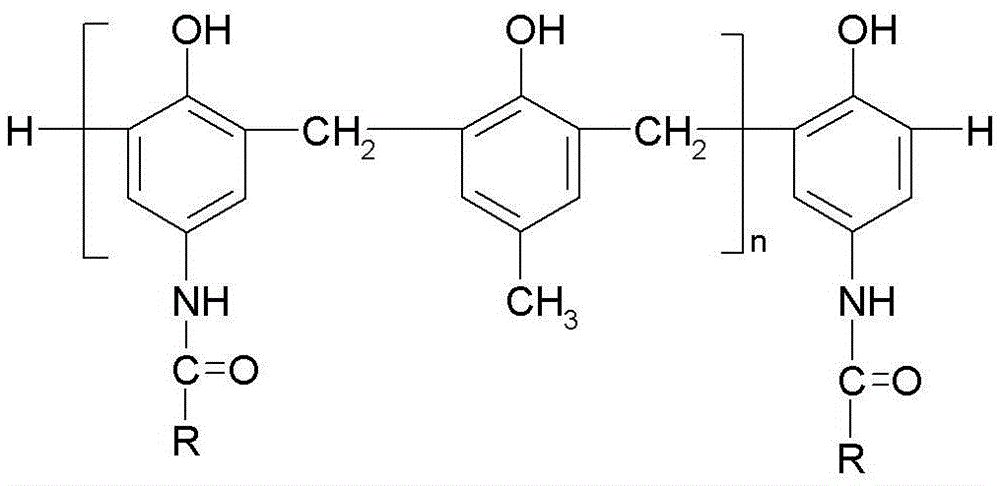
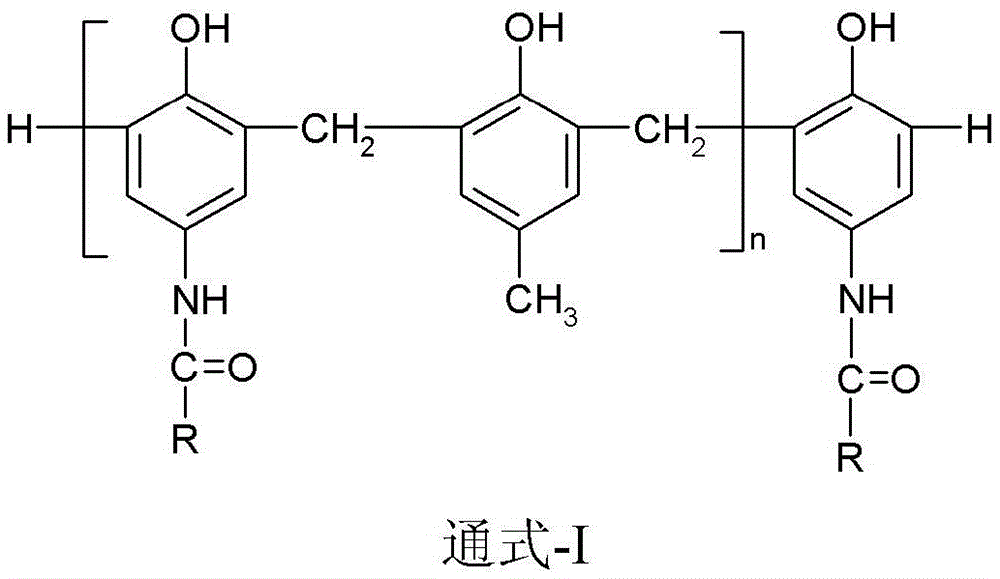
Photosensitive imaging composition containing amide phenolic compound or its oligomer
An imaging composition and compound technology, applied in the field of photosensitive materials, can solve the problems of decreased ink affinity, inability to reduce other properties of the plate, loss of ink affinity of the plate, etc., and achieves light solubility, isopropanol resistance and Good alkali resistance and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0059] Add 75g of glacial acetic acid to a 250ml three-necked round-bottomed flask equipped with mechanical stirring, a thermometer, and a reflux condenser. After stirring and heating up to 60-70°C, add 50.5g (0.5mol) of p-aminophenol within 5-10min. -20min to heat up to 115-125°C and maintain the reaction at this temperature for 5-6h, add 70ml of deionized water after cooling to room temperature, a purple solid precipitates, filter with suction, wash with water until neutral, dry at 50°C, and finally obtain light purple Fine granular product C-A, yield 61.9%.
Synthetic example 2
[0061] Add 150g of glacial acetic acid and 150g of saturated sodium acetate aqueous solution into a 500ml three-necked round-bottomed flask equipped with mechanical stirring and a thermometer, add 45g (0.413mol) of p-aminophenol under stirring, and place it in an ice-water bath to cool down after it is almost completely dissolved. to 0-5°C, and then began to drop 46.6g (0.413mol) of chloroacetyl chloride, the temperature was maintained at 0-5°C during the dropwise addition, and the dropwise addition was completed within 1.5-2.0h. Afterwards, the reaction was maintained at 20-30°C for 2-3h, and after cooling, it was directly suction-filtered, washed with water until neutral, and dried at 50°C to obtain the white powdery solid product C-B with a yield of 69.3%.
Synthetic example 3
[0063] 46.6g (0.413mol) of chloroacetyl chloride in Synthesis Example 2 was replaced by 60.8g (0.413mol) of dichloroacetyl chloride, and the remaining reaction and treatment conditions were unchanged to obtain the white powdery solid product C-C with a yield of 67.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com